Hantzsch Synthesis of the Calcium Channel Blockers Nifedipine, Diludine, and Nitrendipine Using Methanesulfonic Acid as Catalyst
IF 0.8
4区 化学
Q4 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
A simple and efficient one-pot three-component method has been developed and applied for the preparation of the calcium channel blockers nifedipine, diludine, and nitrendipine via Hantzsch dihydropyridine synthesis in good yields employing methanesulfonic acid as catalyst. The protocol stands out for mild reaction conditions, shorter reaction time, high yield, and easy workup and purification procedures, which enhance its practicality. Furthermore, the use of inexpensive and greener catalyst contributes to the efficacy and feasibility of the presented synthetic approach.

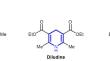
以甲磺酸为催化剂合成钙通道阻滞剂硝苯地平、地鲁定和尼群地平的汉兹合成法
摘要 以甲磺酸为催化剂,通过汉茨二氢吡啶合成法制备钙通道阻滞剂硝苯地平、地鲁定和硝伦地平,建立了一种简单高效的一锅三组分法。该方法具有反应条件温和、反应时间短、产率高、易于操作和纯化等优点,因而实用性更强。此外,使用廉价且更环保的催化剂也提高了该合成方法的有效性和可行性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
1.40
自引率
25.00%
发文量
139
审稿时长
3-6 weeks
期刊介绍:
Russian Journal of Organic Chemistry is an international peer reviewed journal that covers all aspects of modern organic chemistry including organic synthesis, theoretical organic chemistry, structure and mechanism, and the application of organometallic compounds in organic synthesis.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


