Rearrangement of Proline Complexes with Zn2+: An Infrared Multiple Photon Dissociation and Theoretical Investigation
IF 3.1
2区 化学
Q2 BIOCHEMICAL RESEARCH METHODS
Journal of the American Society for Mass Spectrometry
Pub Date : 2024-09-11
DOI:10.1021/jasms.4c00321
引用次数: 0
Abstract
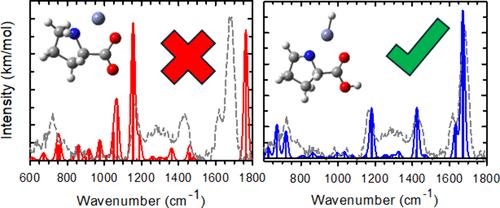
Complexes of proline (Pro) cationized with Zn2+ and Cd2+ were examined by infrared multiple photon dissociation (IRMPD) action spectroscopy using light generated from a free electron laser. Complexes of intact Pro with CdCl+, CdCl+(Pro), a complex of (Zn+Pro-H)+ where a proton has been lost, as well as Zn+(Pro-H)(Pro) were formed by electrospray ionization. In order to identify the structures formed experimentally, the IRMPD spectra were compared to those calculated from optimized structures at the B3LYP/6-311+G(d,p) level for zinc complexes and B3LYP/def2-TZVP level with an effective core potential on cadmium for the CdCl+(Pro) system. For the latter complex, the main binding motif observed has a zwitterionic proline ligand structure, [CO2–]cc, where the metal binds to the two carboxylate oxygens. In contrast, for Zn+(Pro-H)(Pro), both ligands interact with zinc via a [N,CO–][N,CO] binding motif, where binding is observed at the carbonyl oxygens and nitrogens for both ligands, consistent with previous work. In both cases, contributions from different puckers of the proline ring may contribute. For (Zn+Pro-H)+, we identify that the structure is actually ZnH+(Pro-2H), in which the proline has been dehydrogenated and one of the hydrogens has migrated to form a covalent bond with Zn, which verifies a previous report relying on a single OH stretch band.
脯氨酸与 Zn2+ 复合物的重排:红外多光子解离与理论研究
利用自由电子激光器产生的光,通过红外多光子解离(IRMPD)作用光谱对脯氨酸(Pro)与 Zn2+ 和 Cd2+ 阳离子化的络合物进行了研究。通过电喷雾电离形成了完整 Pro 与 CdCl+、CdCl+(Pro)、失去一个质子的 (Zn+Pro-H)+ 复合物以及 Zn+(Pro-H)(Pro)的络合物。为了确定实验形成的结构,将红外分光光度计光谱与在 B3LYP/6-311+G(d,p) 水平下计算出的锌配合物优化结构和在 B3LYP/def2-TZVP 水平下计算出的 CdCl+(Pro)系统镉有效核心电位的优化结构进行了比较。对于后一种配合物,观察到的主要结合模式是一种齐聚脯氨酸配体结构 [CO2-]cc,其中金属与两个羧酸氧原子结合。相反,对于 Zn+(Pro-H)(Pro),两种配体都通过[N,CO-][N,CO]结合基团与锌相互作用,在这两种配体的羰基氧原子和硝基上都观察到了结合,这与之前的研究结果一致。在这两种情况下,脯氨酸环的不同皱褶都可能产生作用。对于 (Zn+Pro-H)+,我们发现该结构实际上是 ZnH+(Pro-2H),其中的脯氨酸已经脱氢,其中一个氢迁移到与 Zn 形成共价键,这验证了之前依赖于单个羟基伸展带的报告。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
5.50
自引率
9.40%
发文量
257
审稿时长
1 months
期刊介绍:
The Journal of the American Society for Mass Spectrometry presents research papers covering all aspects of mass spectrometry, incorporating coverage of fields of scientific inquiry in which mass spectrometry can play a role.
Comprehensive in scope, the journal publishes papers on both fundamentals and applications of mass spectrometry. Fundamental subjects include instrumentation principles, design, and demonstration, structures and chemical properties of gas-phase ions, studies of thermodynamic properties, ion spectroscopy, chemical kinetics, mechanisms of ionization, theories of ion fragmentation, cluster ions, and potential energy surfaces. In addition to full papers, the journal offers Communications, Application Notes, and Accounts and Perspectives

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


