Beauvericin Reverses Epithelial-to-Mesenchymal Transition in Triple-Negative Breast Cancer Cells through Regulation of Notch Signaling and Autophagy
引用次数: 0
Abstract
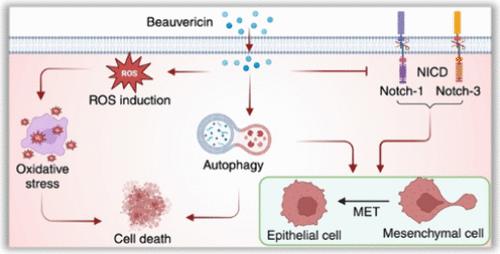
Metastasis stands as a prime contributor to triple-negative breast cancer (TNBC) associated mortality worldwide, presenting heightened severity and significant challenges due to limited treatment options. Addressing TNBC metastasis necessitates innovative approaches and novel therapeutics to specifically target its propensity for dissemination to distant organs. Targeted therapies capable of reversing epithelial-to-mesenchymal transition (EMT) play a crucial role in suppressing metastasis and enhancing the treatment response. Beauvericin, a promising fungal secondary metabolite, exhibits significant potential in diminishing the viability of EMT-induced TNBC cells by triggering intracellular oxidative stress, as evidenced by an enhanced reactive oxygen species level and reduced mitochondrial transmembrane potential. In monolayer cultures, it has exhibited an IC50 of 2.3 μM in both MDA-MB-468 and MDA-MB-231 cells, while in 3D spheroids, the IC50 values are 9.7 and 7.1 μM, respectively. Beauvericin has also reduced the migratory capability of MDA-MB-468 and MDA-MB-231 cells by 1.5- and 1.7-fold, respectively. Both qRT-PCR and Western blot analysis have shown significant upregulation in the expression of epithelial marker (E-cadherin) and downregulation in the expression of mesenchymal markers (N-cadherin, vimentin, Snail, Slug, and β-catenin), following treatment, indicating reversal of EMT. Furthermore, beauvericin has suppressed the Notch signaling pathway by substantially downregulating Notch-1, Notch-3, Hes-1, and cyclinD3 expression and induced autophagy as observed by elevated expression of autophagy markers LC3 and Beclin-1. In conclusion, beauvericin has successfully downregulated TNBC cell survival by inducing oxidative stress and suppressed their migratory potential by reversing EMT through the inhibition of Notch signaling and activation of autophagy.
蒲公英通过调控Notch信号和自噬逆转三阴性乳腺癌细胞的上皮-间质转化
在全球范围内,转移是导致三阴性乳腺癌(TNBC)相关死亡率的主要因素,由于治疗方案有限,转移的严重性和挑战性也随之增加。解决 TNBC 转移问题需要创新方法和新型疗法,以专门针对其向远处器官扩散的倾向。能够逆转上皮细胞向间质转化(EMT)的靶向疗法在抑制转移和增强治疗反应方面发挥着至关重要的作用。Beauvericin 是一种很有前景的真菌次生代谢产物,它通过引发细胞内氧化应激,在降低 EMT 诱导的 TNBC 细胞的活力方面表现出巨大的潜力,活性氧水平的提高和线粒体跨膜电位的降低就是证明。在单层培养中,它对 MDA-MB-468 和 MDA-MB-231 细胞的 IC50 值均为 2.3 μM,而在三维球状培养中,IC50 值分别为 9.7 和 7.1 μM。蒲公英还能使 MDA-MB-468 和 MDA-MB-231 细胞的迁移能力分别降低 1.5 倍和 1.7 倍。qRT-PCR和Western印迹分析表明,处理后上皮标志物(E-cadherin)的表达明显上调,而间充质标志物(N-cadherin、vimentin、Snail、Slug和β-catenin)的表达则明显下调,这表明EMT发生了逆转。此外,蒲公英苷还通过大幅下调Notch-1、Notch-3、Hes-1和cyclinD3的表达来抑制Notch信号通路,并通过提高自噬标记物LC3和Beclin-1的表达来诱导自噬。总之,蒲公英苷通过诱导氧化应激成功地降低了TNBC细胞的存活率,并通过抑制Notch信号传导和激活自噬逆转了EMT,从而抑制了TNBC细胞的迁移潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


