Single-Atom Co–N4 Sites Mediate C═N Formation via Reductive Coupling of Nitroarenes with Alcohols
引用次数: 0
Abstract
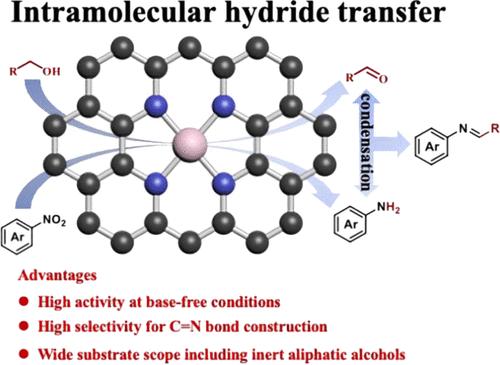
It remains challenging to construct C═N bonds due to their facile hydrogenation. Herein, a single Co atom catalyst was discovered to be active for the selective construction of C═N bonds toward the synthesis of imines and N-heterocycles via reductive coupling of nitroarenes with various alcohols, including inert aliphatic ones. DFT calculations and experimental data revealed that the transfer hydrogenation proceeded via the intramolecular hydride transfer and the transfer of H from the α-Csp3-H bond to the nitro group was the rate-determining step. The single Co atoms served as a bridge to transfer the electrons from the catalyst to the adsorbed alcohol molecules, resulting in the activation of the α-Csp3-H bond. Unlike metal nanoparticles, the C═N bonds in imine products can be reserved due to the large steric hindrance from substituents on C and N. DFT calculation also confirmed that transfer hydrogenation of the C═N bonds in imines is thermodynamically unfavored with a much higher energy barrier compared with the transfer hydrogenation of the –NO2 group (1.47 vs 1.15 eV).
单原子 Co-N4 位点通过硝基烯烃与醇的还原偶联介导 C═N 的形成
由于 C═N 键易于氢化,因此构建 C═N 键仍然具有挑战性。在此,我们发现了一种单 Co 原子催化剂,该催化剂可通过硝基烯烃与各种醇(包括惰性脂肪族醇)的还原偶联,选择性地构建 C═N 键,从而合成亚胺和 N-杂环。DFT 计算和实验数据显示,转移氢化是通过分子内氢化物转移进行的,H 从 α-Csp3-H 键转移到硝基是决定速率的步骤。单个 Co 原子作为桥梁将电子从催化剂转移到吸附的醇分子上,从而激活了 α-Csp3-H 键。DFT 计算还证实,与 -NO2 基团的转移氢化相比,亚胺中 C═N 键的转移氢化在热力学上是不利的,其能量势垒要高得多(1.47 对 1.15 eV)。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


