Hypervalent-Iodine-Mediated Base-Free Oxidative Olefination of Benzylic Amines to Access α,β-Unsaturated Ketones
IF 1.7
4区 化学
Q3 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
We report a one-pot base-free protocol for the oxidative olefination of benzylic amines promoted by a hypervalent iodine reagent for the synthesis of α,β-unsaturated ketones. Mechanistically, (diacetoxyiodo)benzene oxidizes the benzylic amine to the corresponding imine, which, on reaction with a phenacyl(triphenyl)phosphonium bromide salt and an in situ generated acetoxy anion leads to an α,β-unsaturated ketone. A wide range of α,β-unsaturated ketones were easily accessed through direct oxidative olefination of substituted benzylic amines in good to excellent yields and with high E-selectivity.
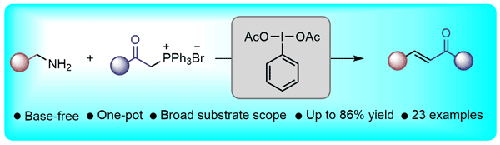
高价碘介导的苄胺无碱氧化烯化反应,以获得 α,β-不饱和酮体
我们报告了一种在高价碘试剂促进下合成α,β-不饱和酮的苄胺氧化烯化反应的单锅无碱方案。从机理上讲,(二乙酰氧基碘)苯会将苄胺氧化成相应的亚胺,亚胺与苯基(三苯基)溴化磷盐和原位生成的乙酰氧基阴离子反应后,会生成α,β-不饱和酮。通过取代苄胺的直接氧化烯化反应,可以很容易地获得多种α,β-不饱和酮,而且产率从良好到极佳,并具有很高的 E 选择性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Synlett
化学-有机化学
CiteScore
3.40
自引率
5.00%
发文量
369
审稿时长
1 months
期刊介绍:
SYNLETT is an international journal reporting research results and current trends in chemical synthesis in short personalized reviews and preliminary communications. It covers all fields of scientific endeavor that involve organic synthesis, including catalysis, organometallic, medicinal, biological, and photochemistry, but also related disciplines and offers the possibility to publish scientific primary data.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


