Direct Synthesis of N-Substituted Phosphinecarboxamides from [TBA][P(SiCl3)2] and Isonitriles
IF 1.4
4区 化学
Q3 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
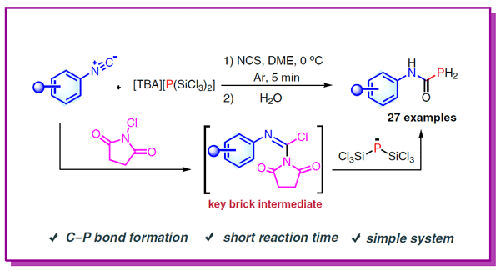
In this investigation, N-substituted phosphinecarboxamides were produced through the reaction of [TBA][P(SiCl3)2] with isonitriles. This method capitalizes on the flexibility of isonitriles as a source of both nitrogen and carbonyl groups, offering a novel route to the generation of PH2-type compounds. This approach is characterized by rapid reaction times, simple procedural requirements, compatibility with a diverse array of substrates, and the conversion of [TBA][P(SiCl3)2] into organic phosphorus compounds. Additionally, we systematically studied the reaction mechanism of isonitrile with [TBA][P(SiCl3)2] through controlled experiments and density functional theory (DFT) calculations.
由 [TBA][P(SiCl3)2]和异腈直接合成 N-取代的膦甲酰胺
在这项研究中,[TBA][P(SiCl3)2] 与异腈反应生成了 N-取代的膦甲酰胺。这种方法利用了异腈作为氮和羰基来源的灵活性,为生成 PH2- 型化合物提供了一条新途径。这种方法的特点是反应时间快、程序要求简单、与多种底物兼容,并能将 [TBA][P(SiCl3)2] 转化为有机磷化合物。此外,我们还通过对照实验和密度泛函理论 (DFT) 计算系统地研究了异腈与 [TBA][P(SiCl3)2]的反应机理。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Synlett
化学-有机化学
CiteScore
3.40
自引率
5.00%
发文量
369
审稿时长
1 months
期刊介绍:
SYNLETT is an international journal reporting research results and current trends in chemical synthesis in short personalized reviews and preliminary communications. It covers all fields of scientific endeavor that involve organic synthesis, including catalysis, organometallic, medicinal, biological, and photochemistry, but also related disciplines and offers the possibility to publish scientific primary data.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


