RBM25 is required to restrain inflammation via ACLY RNA splicing-dependent metabolism rewiring
IF 19.8
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
Abstract
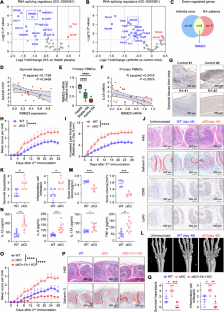
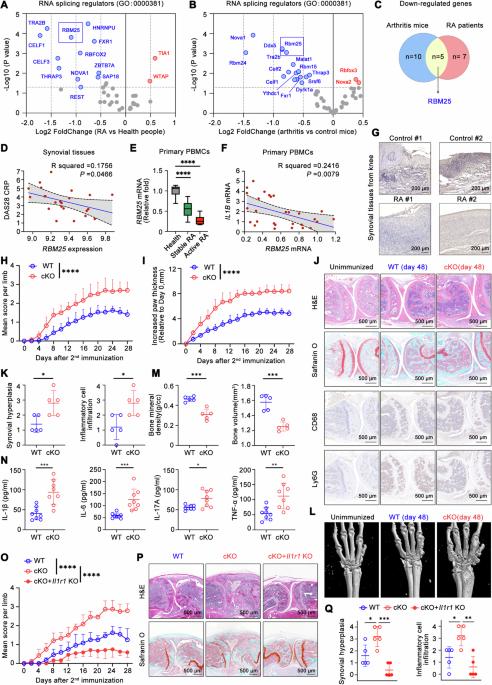
Spliceosome dysfunction and aberrant RNA splicing underline unresolved inflammation and immunopathogenesis. Here, we revealed the misregulation of mRNA splicing via the spliceosome in the pathogenesis of rheumatoid arthritis (RA). Among them, decreased expression of RNA binding motif protein 25 (RBM25) was identified as a major pathogenic factor in RA patients and experimental arthritis mice through increased proinflammatory mediator production and increased hyperinflammation in macrophages. Multiomics analyses of macrophages from RBM25-deficient mice revealed that the transcriptional enhancement of proinflammatory genes (including Il1b, Il6, and Cxcl10) was coupled with histone 3 lysine 9 acetylation (H3K9ac) and H3K27ac modifications as well as hypoxia inducible factor-1α (HIF-1α) activity. Furthermore, RBM25 directly bound to and mediated the 14th exon skipping of ATP citrate lyase (Acly) pre-mRNA, resulting in two distinct Acly isoforms, Acly Long (Acly L) and Acly Short (Acly S). In proinflammatory macrophages, Acly L was subjected to protein lactylation on lysine 918/995, whereas Acly S did not, which influenced its affinity for metabolic substrates and subsequent metabolic activity. RBM25 deficiency overwhelmingly increased the expression of the Acly S isoform, enhancing glycolysis and acetyl-CoA production for epigenetic remodeling, macrophage overactivation and tissue inflammatory injury. Finally, macrophage-specific deletion of RBM25 led to inflammaging, including spontaneous arthritis in various joints of mice and inflammation in multiple organs, which could be relieved by pharmacological inhibition of Acly. Overall, targeting the RBM25-Acly splicing axis represents a potential strategy for modulating macrophage responses in autoimmune arthritis and aging-associated inflammation.
需要 RBM25 通过 ACLY RNA 剪接依赖性新陈代谢重新布线来抑制炎症
剪接体功能障碍和 RNA 剪接异常凸显了尚未解决的炎症和免疫发病机制。在这里,我们揭示了类风湿性关节炎(RA)发病机制中通过剪接体对 mRNA 剪接的误调。其中,RNA 结合基调蛋白 25(RBM25)表达的减少被确定为 RA 患者和实验性关节炎小鼠的主要致病因素,它通过增加促炎介质的产生和巨噬细胞中的高炎症性增加而致病。对 RBM25 缺陷小鼠巨噬细胞的多组学分析表明,促炎基因(包括 Il1b、Il6 和 Cxcl10)的转录增强与组蛋白 3 赖氨酸 9 乙酰化(H3K9ac)和 H3K27ac 修饰以及缺氧诱导因子-1α(HIF-1α)活性有关。此外,RBM25直接与ATP柠檬酸裂解酶(Acly)前mRNA的第14个外显子结合并介导其跳过,从而产生了两种不同的Acly异构体,即Acly长(Acly L)和Acly短(Acly S)。在促炎巨噬细胞中,Acly L 在赖氨酸 918/995 上发生蛋白乳化,而 Acly S 则没有,这影响了它对代谢底物的亲和力和随后的代谢活性。RBM25 缺乏会显著增加 Acly S 异构体的表达,从而提高糖酵解和乙酰-CoA 的产生,促进表观遗传重塑、巨噬细胞过度活化和组织炎症损伤。最后,巨噬细胞特异性缺失 RBM25 会导致炎症,包括小鼠各种关节的自发性关节炎和多个器官的炎症,而药物抑制 Acly 可以缓解这些炎症。总之,靶向RBM25-Acly剪接轴是调节自身免疫性关节炎和衰老相关炎症中巨噬细胞反应的一种潜在策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
31.20
自引率
1.20%
发文量
903
审稿时长
1 months
期刊介绍:
Cellular & Molecular Immunology, a monthly journal from the Chinese Society of Immunology and the University of Science and Technology of China, serves as a comprehensive platform covering both basic immunology research and clinical applications. The journal publishes a variety of article types, including Articles, Review Articles, Mini Reviews, and Short Communications, focusing on diverse aspects of cellular and molecular immunology.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


