Rack1 regulates B-cell development and function by binding to and stabilizing the transcription factor Pax5
IF 21.8
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
Abstract
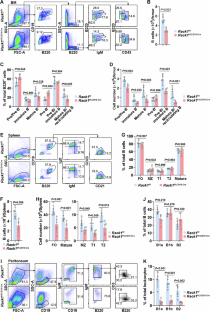
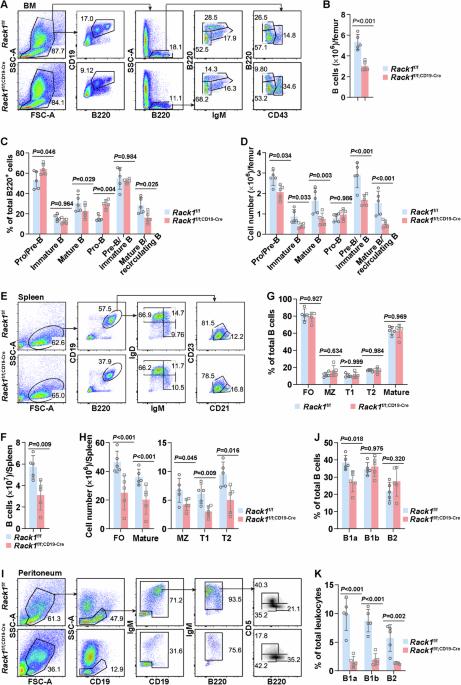
The transcription factor Pax5 activates genes essential for B-cell development and function. However, the regulation of Pax5 expression remains elusive. The adaptor Rack1 can interact with multiple transcription factors and modulate their activation and/or stability. However, its role in the transcriptional control of B-cell fates is largely unknown. Here, we show that CD19-driven Rack1 deficiency leads to pro-B accumulation and a simultaneous reduction in B cells at later developmental stages. The generation of bone marrow chimeras indicates a cell-intrinsic role of Rack1 in B-cell homeostasis. Moreover, Rack1 augments BCR and TLR signaling in mature B cells. On the basis of the aberrant expression of Pax5-regulated genes, including CD19, upon Rack1 deficiency, further exploration revealed that Rack1 maintains the protein level of Pax5 through direct interaction and consequently prevents Pax5 ubiquitination. Accordingly, Mb1-driven Rack1 deficiency almost completely blocks B-cell development at the pro-B-cell stage. Ectopic expression of Pax5 in Rack1-deficient pro-B cells partially rescues B-cell development. Thus, Rack1 regulates B-cell development and function through, at least partially, binding to and stabilizing Pax5.
Rack1 通过与转录因子 Pax5 结合并使其稳定来调控 B 细胞的发育和功能
转录因子 Pax5 可激活 B 细胞发育和功能所必需的基因。然而,Pax5 的表达调控仍然难以捉摸。适配体 Rack1 可与多种转录因子相互作用,并调节它们的激活和/或稳定性。然而,它在B细胞命运转录调控中的作用在很大程度上还不为人所知。在这里,我们发现 CD19 驱动的 Rack1 缺乏会导致亲 B 细胞的积累,并同时导致发育后期 B 细胞的减少。骨髓嵌合体的产生表明 Rack1 在 B 细胞稳态中发挥着细胞内在的作用。此外,Rack1 还能增强成熟 B 细胞中的 BCR 和 TLR 信号转导。基于 Rack1 缺乏时包括 CD19 在内的 Pax5 调控基因的异常表达,进一步研究发现 Rack1 通过直接相互作用维持 Pax5 的蛋白水平,从而阻止 Pax5 泛素化。因此,Mb1 驱动的 Rack1 缺乏几乎完全阻断了前 B 细胞阶段的 B 细胞发育。在 Rack1 缺乏的原 B 细胞中异位表达 Pax5 可部分挽救 B 细胞的发育。因此,Rack1 至少部分通过与 Pax5 结合并稳定 Pax5 来调节 B 细胞的发育和功能。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
31.20
自引率
1.20%
发文量
903
审稿时长
1 months
期刊介绍:
Cellular & Molecular Immunology, a monthly journal from the Chinese Society of Immunology and the University of Science and Technology of China, serves as a comprehensive platform covering both basic immunology research and clinical applications. The journal publishes a variety of article types, including Articles, Review Articles, Mini Reviews, and Short Communications, focusing on diverse aspects of cellular and molecular immunology.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


