Cascade assembling of aldehydes and two molecules of dimedone into 4H-spiro[1-benzofuran-2,1'-cyclohexane]-2',4,6'-triones under column chromatography-free protocol at room temperature
IF 1
4区 化学
Q3 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
A new type of one-pot Knoevenagel–Michael reaction with the following NBS-induced cyclization was found: a direct one-pot transformation of aldehydes and two molecules of dimedone into substituted 4H-spiro[1-benzofuran-2,1'-cyclohexane]-2',4,6'-triones in 86–95% yields. This one-pot process is a very efficient and convenient way to access substituted 4H-spiro[1-benzofuran-2,1'-cyclohexane]-2',4,6'-triones – useful compounds for different biomedical applications with reasonable and nonexpensive starting materials. Mild and facile conditions of this chemical cascade one-pot process, as well as non-chromatographic isolation procedure lead to excellent substance yields.
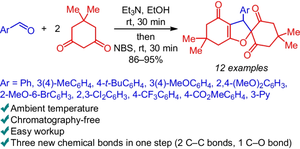
在室温无柱色谱条件下,醛和两分子二甲基酮级联组装成 4H-螺[1-苯并呋喃-2,1'-环己烷]-2',4,6'-三酮
研究人员发现了一种新型的 NBS 诱导环化的一锅式 Knoevenagel-Michael 反应:将醛和两分子二甲基酮直接一锅式转化为取代的 4H-螺[1-苯并呋喃-2,1'-环己烷]-2',4,6'-三酮,收率为 86-95%。这种一锅法是获得取代的 4H-螺[1-苯并呋喃-2,1'-环己烷]-2',4,6'-三酮类化合物的一种非常有效和方便的方法。这种化学级联一锅法的条件温和、操作简便,而且采用非色谱分离程序,因此物质收率极高。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
2.90
自引率
13.30%
发文量
98
审稿时长
1 months
期刊介绍:
The international journal Chemistry of Heterocyclic Compounds publishes original papers, short communications, reviews, and mini-reviews dealing with problems in the field of heterocyclic chemistry in Russian and English. The Journal also publishes reviews and annotations on new books and brief reports on conferences in the field of heterocyclic chemistry, as well as commemoratives dedicated to prominent heterocyclic chemists.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


