Design, synthesis, and anti-inflammatory activity of novel 20-O-substituted camptothecin carbamate derivatives
IF 1
4区 化学
Q3 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
Twelve novel camptothecin derivatives with a carbamate substituent at position 20 were designed and synthesized. Most of the targeted compounds indicated potent anti-inflammatory activity in a lipopolysaccharide stimulation of peritoneal macrophage model. Among them, the benzyl(methyl)amine derivative showed the most pronounced anti-inflammatory activity at a concentration of 1 μM and TNF-α inhibitory rate is increased by 10.2% compared to the lead compound (N,N-dimethylethylenediamine derivative).
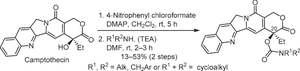
新型 20-O 取代喜树碱氨基甲酸酯衍生物的设计、合成和抗炎活性
研究人员设计并合成了 12 种新型喜树碱衍生物,这些衍生物的第 20 位具有氨基甲酸酯取代基。在脂多糖刺激腹腔巨噬细胞模型中,大多数目标化合物都显示出了强大的抗炎活性。其中,苄基(甲基)胺衍生物在 1 μM 浓度下表现出最明显的抗炎活性,与先导化合物(N,N-二甲基乙二胺衍生物)相比,TNF-α 抑制率提高了 10.2%。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
2.90
自引率
13.30%
发文量
98
审稿时长
1 months
期刊介绍:
The international journal Chemistry of Heterocyclic Compounds publishes original papers, short communications, reviews, and mini-reviews dealing with problems in the field of heterocyclic chemistry in Russian and English. The Journal also publishes reviews and annotations on new books and brief reports on conferences in the field of heterocyclic chemistry, as well as commemoratives dedicated to prominent heterocyclic chemists.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


