A Comprehensive Approach to C3a-Aryl-Substituted Hydroindole Alkaloids by Utilizing Enantioselective Gold Catalysis
引用次数: 0
Abstract
A diversity-oriented total synthesis for Amaryllidaceae alkaloids incorporating the frequently found C3a-arylated hydroindole moiety was developed. Chiral-anion-induced gold(I) catalysis was employed for the cyclization of 1,4-diynes to the pyrrolidine and the installation of the all-carbon quaternary stereocenter. Both enantiomeric series of crinine-type alkaloids in high enantiopurity were accessible by this methodology. The formal synthesis of a wide range of Amaryllidaceae alkaloids is described, such as (+)-vitattine, (–)-epi-vitattine, (–)-elwesein, (–)-epi-elwesein, (–)-crinine, (–)-epi-crinine, (–)-buphanisine, (–)-flexinine, and (+)-gracilamine.
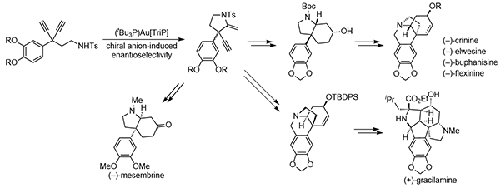
利用对映体选择性金催化生产 C3a-芳基取代的氢吲哚生物碱的综合方法
研究人员开发了一种以多样性为导向的金盏花科生物碱全合成方法,其中包含了经常发现的 C3a-芳基化氢吲哚分子。手性阴离子诱导的金(I)催化被用于 1,4-二炔到吡咯烷的环化以及全碳季立体中心的安装。通过这种方法,可以获得对映体纯度很高的两种对映体系列的奎宁类生物碱。本文介绍了多种金盏花科生物碱的正式合成方法,如(+)-vitattine、(-)-epi-vitattine、(-)-elwesein、(-)-epi-elwesein、(-)-crinine、(-)-epi-crinine、(-)-buphanisine、(-)-flexinine和(+)-gracilamine。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


