USP33 facilitates the ovarian cancer progression via deubiquitinating and stabilizing CBX2
IF 6.9
1区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Post-translational modifications of proteins play a pivotal role in both the initiation and progression of ovarian cancer. Despite the recognition of USP33 as a significant factor in various cancers, its specific function and underlying mechanisms in ovarian cancer remain elusive. Proteomics and ubiquitinomics approaches were coupled to screen novel substrate proteins directly regulated by USP33. Our findings unveil that USP33 was observed to eliminate K27- and K48-linked ubiquitin chains from CBX2 at the K277 position. Notably, acetylation of CBX2 at K199, catalyzed by lysine acetyltransferase GCN5, was found to enhance its interaction with USP33, subsequently promoting further deubiquitination and stabilization. Functionally, our experiments demonstrate that USP33 significantly enhances ovarian cancer proliferation and metastasis in a CBX2-dependent manner. Furthermore, analysis revealed a direct positive correlation between the expression levels of USP33 and CBX2 proteins in human specimens, with elevated levels being associated with reduced survival rates in ovarian cancer patients. These findings elucidate the mechanism by which USP33 augments ovarian cancer progression through the stabilization of CBX2, underscoring the USP33-CBX2 axis as a promising therapeutic target in ovarian cancer management.
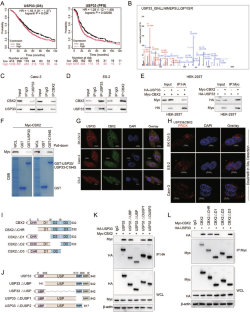
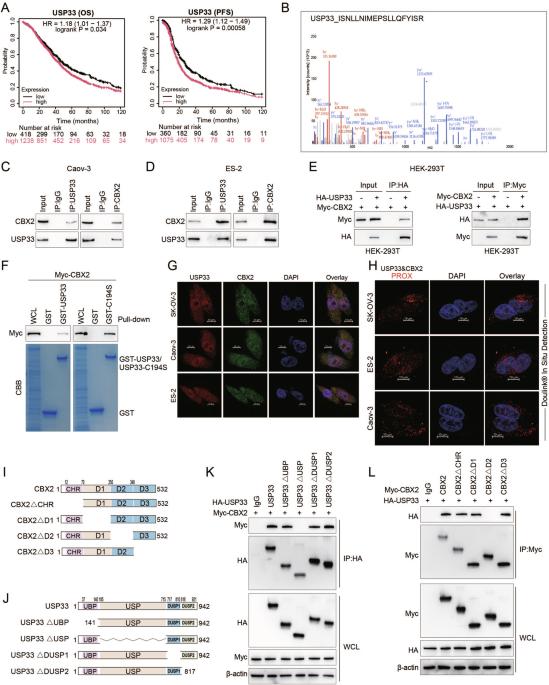
USP33 通过去泛素化和稳定 CBX2 促进卵巢癌的进展
蛋白质的翻译后修饰在卵巢癌的发生和发展中起着至关重要的作用。尽管 USP33 被认为是多种癌症中的一个重要因素,但它在卵巢癌中的具体功能和潜在机制仍然难以捉摸。我们将蛋白质组学和泛素组学方法结合起来,筛选出受 USP33 直接调控的新型底物蛋白。我们的研究结果表明,USP33 可在 K277 位消除 CBX2 上与 K27 和 K48 链接的泛素链。值得注意的是,在赖氨酸乙酰转移酶 GCN5 的催化下,CBX2 在 K199 处的乙酰化被发现会增强其与 USP33 的相互作用,从而促进进一步的去泛素化和稳定化。在功能上,我们的实验证明,USP33 能以 CBX2 依赖性方式显著增强卵巢癌的增殖和转移。此外,分析表明,USP33 和 CBX2 蛋白在人体标本中的表达水平呈直接正相关,水平升高与卵巢癌患者生存率降低有关。这些发现阐明了 USP33 通过稳定 CBX2 促进卵巢癌进展的机制,强调 USP33-CBX2 轴是治疗卵巢癌的一个有前景的治疗靶点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Oncogene
医学-生化与分子生物学
CiteScore
15.30
自引率
1.20%
发文量
404
审稿时长
1 months
期刊介绍:
Oncogene is dedicated to advancing our understanding of cancer processes through the publication of exceptional research. The journal seeks to disseminate work that challenges conventional theories and contributes to establishing new paradigms in the etio-pathogenesis, diagnosis, treatment, or prevention of cancers. Emphasis is placed on research shedding light on processes driving metastatic spread and providing crucial insights into cancer biology beyond existing knowledge.
Areas covered include the cellular and molecular biology of cancer, resistance to cancer therapies, and the development of improved approaches to enhance survival. Oncogene spans the spectrum of cancer biology, from fundamental and theoretical work to translational, applied, and clinical research, including early and late Phase clinical trials, particularly those with biologic and translational endpoints.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


