AR coactivators, CBP/p300, are critical mediators of DNA repair in prostate cancer
IF 6.9
1区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
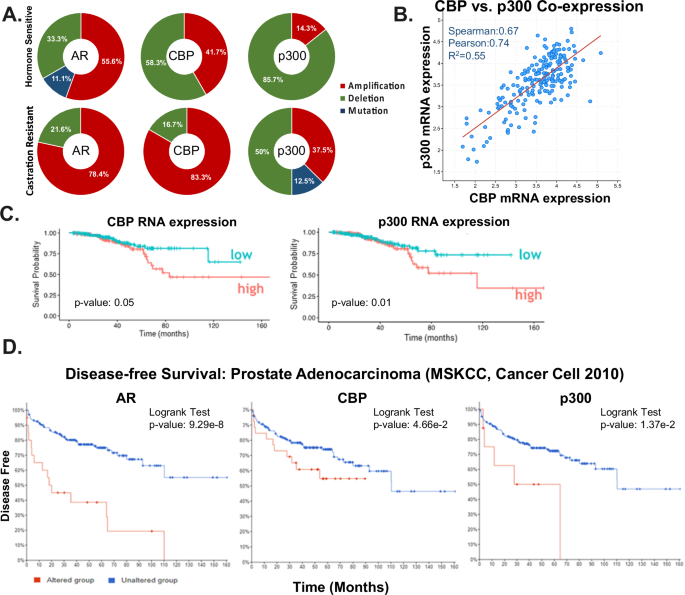
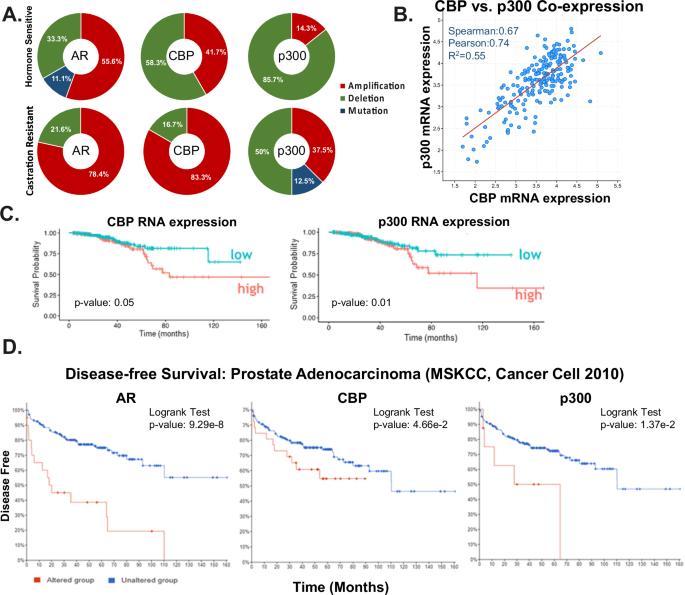
Castration resistant prostate cancer (CRPC) remains an incurable disease stage with ineffective treatments options. Here, the androgen receptor (AR) coactivators CBP/p300, which are histone acetyltransferases, were identified as critical mediators of DNA damage repair (DDR) to potentially enhance therapeutic targeting of CRPC. Key findings demonstrate that CBP/p300 expression increases with disease progression and selects for poor prognosis in metastatic disease. CBP/p300 bromodomain inhibition enhances response to standard of care therapeutics. Functional studies, CBP/p300 cistrome mapping, and transcriptome in CRPC revealed that CBP/p300 regulates DDR. Further mechanistic investigation showed that CBP/p300 attenuation via therapeutic targeting and genomic knockdown decreases homologous recombination (HR) factors in vitro, in vivo, and in human prostate cancer (PCa) tumors ex vivo. Similarly, CBP/p300 expression in human prostate tissue correlates with HR factors. Lastly, targeting CBP/p300 impacts HR-mediate repair and patient outcome. Collectively, these studies identify CBP/p300 as drivers of PCa tumorigenesis and lay the groundwork to optimize therapeutic strategies for advanced PCa via CBP/p300 inhibition, potentially in combination with AR-directed and DDR therapies.
AR 辅激活因子 CBP/p300 是前列腺癌 DNA 修复的关键介质
阉割抵抗性前列腺癌(CRPC)仍然是一种无法治愈的疾病,治疗效果不佳。在这里,作为组蛋白乙酰转移酶的雄激素受体(AR)辅助激活剂CBP/p300被确定为DNA损伤修复(DDR)的关键介质,从而有可能加强针对CRPC的治疗。主要研究结果表明,CBP/p300的表达会随着疾病的进展而增加,并选择性地影响转移性疾病的不良预后。抑制CBP/p300溴链可增强对标准疗法的反应。功能研究、CBP/p300 cistrome图谱和CRPC的转录组显示,CBP/p300调节DDR。进一步的机理研究表明,通过治疗靶向和基因组敲除削弱 CBP/p300,可减少体外、体内和体外人类前列腺癌(PCa)肿瘤中的同源重组(HR)因子。同样,CBP/p300 在人类前列腺组织中的表达也与 HR 因子相关。最后,靶向 CBP/p300 会影响 HR 直接修复和患者预后。总之,这些研究确定了 CBP/p300 是 PCa 肿瘤发生的驱动因素,并为通过抑制 CBP/p300 优化晚期 PCa 的治疗策略奠定了基础,CBP/p300 有可能与 AR 定向疗法和 DDR 疗法相结合。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Oncogene
医学-生化与分子生物学
CiteScore
15.30
自引率
1.20%
发文量
404
审稿时长
1 months
期刊介绍:
Oncogene is dedicated to advancing our understanding of cancer processes through the publication of exceptional research. The journal seeks to disseminate work that challenges conventional theories and contributes to establishing new paradigms in the etio-pathogenesis, diagnosis, treatment, or prevention of cancers. Emphasis is placed on research shedding light on processes driving metastatic spread and providing crucial insights into cancer biology beyond existing knowledge.
Areas covered include the cellular and molecular biology of cancer, resistance to cancer therapies, and the development of improved approaches to enhance survival. Oncogene spans the spectrum of cancer biology, from fundamental and theoretical work to translational, applied, and clinical research, including early and late Phase clinical trials, particularly those with biologic and translational endpoints.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


