Multifocal, multiphenotypic tumours arising from an MTOR mutation acquired in early embryogenesis
IF 6.9
1区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
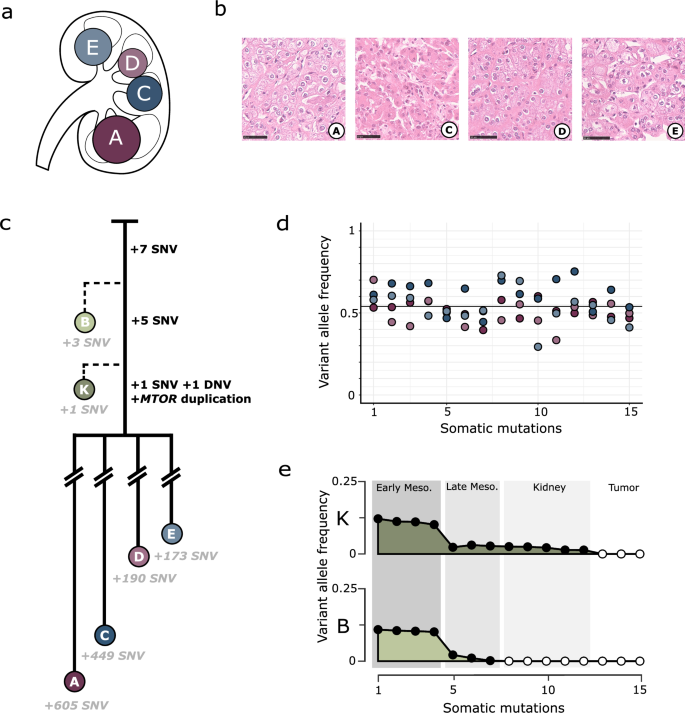
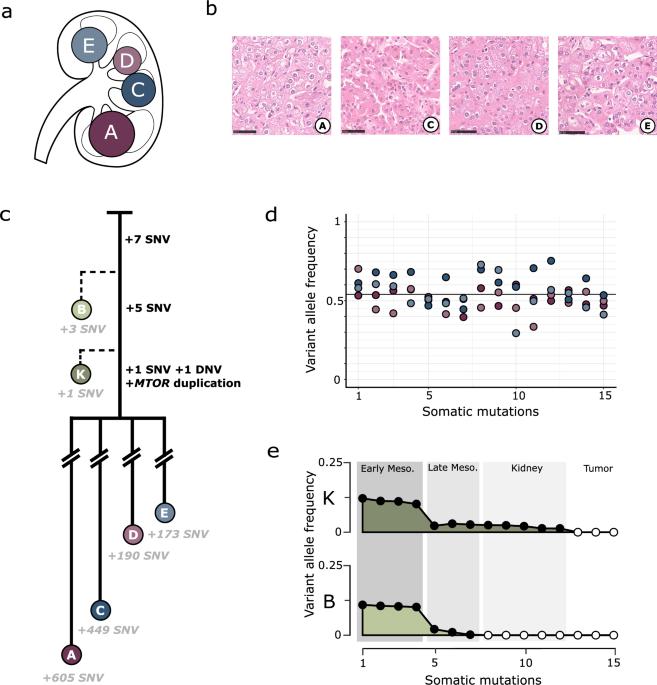
Embryogenesis is a vulnerable time. Mutations in developmental cells can result in the wide dissemination of cells predisposed to disease within mature organs. We characterised the evolutionary history of four synchronous renal tumours from a 14-year-old girl using whole genome sequencing alongside single cell and bulk transcriptomic sequencing. Phylogenetic reconstruction timed the origin of all tumours to a multipotent embryonic cell committed to the right kidney, around 4 weeks post-conception. Biochemical and structural analysis of their shared MTOR mutation, absent from normal tissues, demonstrates enhanced protein flexibility, enabling a FAT domain hinge to dramatically increase activity of mTORC1 and mTORC2. Developmental mutations, not usually detected in traditional genetic screening, have vital clinical importance in guiding prognosis, targeted treatment, and family screening decisions for paediatric tumours.
胚胎早期获得的 MTOR 基因突变引发的多灶、多型性肿瘤
胚胎发育是一个脆弱的时期。发育细胞中的突变会导致易患病细胞在成熟器官中广泛传播。我们利用全基因组测序以及单细胞和大体转录组测序,研究了一名14岁女孩的四个同步肾肿瘤的进化史。系统发育重建将所有肿瘤的起源时间定为受孕后 4 周左右的右肾多能胚胎细胞。对正常组织中不存在的共同MTOR突变进行的生化和结构分析表明,该突变增强了蛋白质的灵活性,使FAT结构域铰链能够显著提高mTORC1和mTORC2的活性。发育突变通常不会在传统的基因筛查中被检测到,但它在指导儿科肿瘤的预后、靶向治疗和家庭筛查决策方面具有重要的临床意义。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Oncogene
医学-生化与分子生物学
CiteScore
15.30
自引率
1.20%
发文量
404
审稿时长
1 months
期刊介绍:
Oncogene is dedicated to advancing our understanding of cancer processes through the publication of exceptional research. The journal seeks to disseminate work that challenges conventional theories and contributes to establishing new paradigms in the etio-pathogenesis, diagnosis, treatment, or prevention of cancers. Emphasis is placed on research shedding light on processes driving metastatic spread and providing crucial insights into cancer biology beyond existing knowledge.
Areas covered include the cellular and molecular biology of cancer, resistance to cancer therapies, and the development of improved approaches to enhance survival. Oncogene spans the spectrum of cancer biology, from fundamental and theoretical work to translational, applied, and clinical research, including early and late Phase clinical trials, particularly those with biologic and translational endpoints.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


