The chemical nature of SO2 poisoning of Cu-CHA-based SCR catalysts for NOx removal in diesel exhausts†
IF 4.2
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
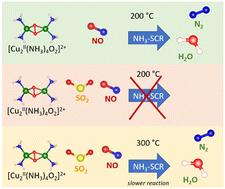
This study addresses the impact of SO2 exposure on the catalytic performance of a Cu-chabazite-based SCR catalyst, as used in diesel exhausts, to reduce the emission of NOx through the NH3-SCR reaction. The SCR activity is determined by a reaction of NO with the [Cu2II(NH3)4O2]2+ intermediate. The same intermediate is also the most reactive Cu-species towards SO2. We demonstrate here that the reaction with NO at 200 °C is limited after exposure of the [Cu2II(NH3)4O2]2+ complex to SO2 or SO2/O2. Heating the catalyst to 300 °C in NO restores the reaction, albeit at a significantly lower rate. The lower reactivity towards NO indicates that exposure of [Cu2II(NH3)4O2]2+ to SO2 induces changes in the chemistry of Cu in the catalyst. This implies that poisoning of Cu-chabazite catalysts by SO2 is, at least in part, of the chemical nature, and may be not limited to the physical pore blocking.
用于去除柴油机废气中氮氧化物的 Cu-CHA 型 SCR 催化剂的二氧化硫中毒化学本质
本研究探讨了二氧化硫暴露对基于铜-茶钒石的 SCR 催化剂催化性能的影响,该催化剂用于柴油机尾气中,通过 NH3-SCR 反应减少氮氧化物的排放。SCR 活性由 NO 与 [Cu2II(NH3)4O2]2+ 中间体的反应决定。该中间体也是对二氧化硫反应活性最高的铜种。我们在此证明,[Cu2II(NH3)4O2]2+ 复合物与 SO2 或 SO2/O2 接触后,在 200 ℃ 下与 NO 的反应受到限制。在 NO 中将催化剂加热至 300 °C,可以恢复反应,但反应速率明显降低。对 NO 较低的反应活性表明,[Cu2II(NH3)4O2]2+ 与 SO2 的接触会引起催化剂中 Cu 化学性质的变化。这意味着二氧化硫对铜-钠钙石催化剂的毒害至少部分是化学性的,可能并不局限于物理孔隙堵塞。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Catalysis Science & Technology
CHEMISTRY, PHYSICAL-
CiteScore
8.70
自引率
6.00%
发文量
587
审稿时长
1.5 months
期刊介绍:
A multidisciplinary journal focusing on cutting edge research across all fundamental science and technological aspects of catalysis.
Editor-in-chief: Bert Weckhuysen
Impact factor: 5.0
Time to first decision (peer reviewed only): 31 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


