Nickel–PNN catalysed sustainable synthesis of polysubstituted quinolines under microwave irradiation†
IF 4.2
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
In this manuscript, the synthesis of a series of cationic NiII complexes based on mixed donor triazolyl-pyridine-based aminophosphine, [(NiCl){R′PRNN}-κ3-P,N,N]OTf (R = H or Me; R′ = Ph or tBu), is described. The neutral dearomatized complexes [(NiCl){R′PNN}-κ3-P,N,N] (R′ = Ph or tBu) were prepared by deprotonation of [(NiCl){PPhN(H)N}-κ3-P,N,N]OTf (Ni1) and [(NiCl){(tBu2P)N(H)N}-κ3-P,N,N]OTf (Ni3) with 1 equiv. of tBuOK. These complexes (0.5 mol%) were employed for the synthesis of substituted quinolines at 110 °C under microwave irradiation with 20 mol% of KOH. Among these, complexes [(NiCl){t BuPHNN-κ3-P,N,N}]OTf (Ni3) and [(NiCl){t BuPNN}-κ3-P,N,N] (Ni5) were found to be highly efficient. A wide range of derivatives, including aryl, aliphatic, acyclic ketones, primary alcohols and aminobenzyl alcohols, yielded the corresponding quinolines in good to excellent yields (62 examples). A series of control experiments were carried out to establish the reaction mechanism. HR-MS spectral studies were investigated to characterize the nickel-hydride intermediate. Mechanistic studies confirmed that the reaction takes an acceptorless dehydrogenative coupling pathway.
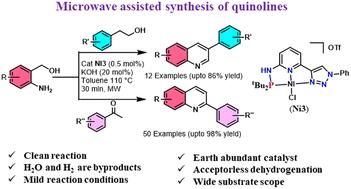
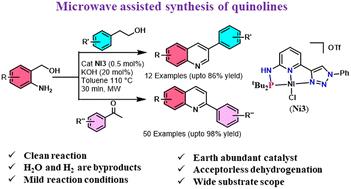
微波辐照下镍-PNN 催化多取代喹啉的可持续合成
本手稿描述了一系列基于混合供体三唑基吡啶基氨基膦的阳离子 NiII 复合物[(NiCl){R′PRNN}-κ3-P,N,N]OTf(R = H 或 Me;R′ = Ph 或 tBu)的合成过程。这些配合物(0.5 摩尔%)在 110 °C、20 摩尔% KOH 的微波辐照下用于合成取代的喹啉类化合物。其中,[(NiCl){tBuPHNN-κ3-P,N,N}]OTf(Ni3)和[(NiCl){tBuPNNN}-κ3-P,N,N](Ni5)是高效的配合物。包括芳基、脂肪族、无环酮、伯醇和氨基苄醇在内的多种衍生物都能以良好到极佳的收率生成相应的喹啉类化合物(62 个实例)。为确定反应机理,进行了一系列对照实验。为确定镍酸酐中间体的特性,还进行了 HR-MS 光谱研究。机理研究证实,该反应采用了无受体脱氢偶联途径。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Catalysis Science & Technology
CHEMISTRY, PHYSICAL-
CiteScore
8.70
自引率
6.00%
发文量
587
审稿时长
1.5 months
期刊介绍:
A multidisciplinary journal focusing on cutting edge research across all fundamental science and technological aspects of catalysis.
Editor-in-chief: Bert Weckhuysen
Impact factor: 5.0
Time to first decision (peer reviewed only): 31 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


