Manipulating anion intercalation into layered double hydroxide for alkaline seawater oxidation at high current density†
IF 4.2
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
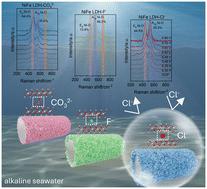
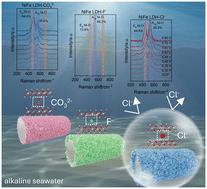
In this work, we propose a convenient strategy to manipulate anion intercalation into layered double hydroxide. The obtained NiFe LDH-Cl− electrode shows outstanding OER performance with both low overpotentials and high stability in alkaline seawater at high current density. Ultra-low overpotentials of 255 mV and 350 mV are required in 1 M KOH and alkaline seawater at a current density of 200 mA cm−2. In addition, NiFe LDH-Cl− can stably operate at 200 mA cm−2 for 100 h. In situ Raman studies reveal that the active γ-NiOOH is generated on NiFe LDH-Cl− at a low potential by surface reconstruction, and Cl− intercalation helps optimize the peak area ratio of Eg and A1g, which can be favorable for the alkaline seawater OER.
操纵阴离子插层到层状双氢氧化物中,实现高电流密度下的碱性海水氧化
在这项工作中,我们提出了一种将阴离子插层到层状双氢氧化物中的便捷策略。所获得的镍铁合金 LDH-Cl- 电极在高电流密度的碱性海水中表现出卓越的 OER 性能,既具有低过电位,又具有高稳定性。在电流密度为 200 mA cm-2 的 1 M KOH 和碱性海水中,过电位分别为 255 mV 和 350 mV。此外,NiFe LDH-Cl- 可在 200 mA cm-2 下稳定运行 100 小时。原位拉曼研究表明,活性 γ-NiOOH 是在低电位下通过表面重构在 NiFe LDH-Cl- 上生成的,Cl- 插层有助于优化 Eg 和 A1g 的峰面积比,这对碱性海水 OER 是有利的。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Catalysis Science & Technology
CHEMISTRY, PHYSICAL-
CiteScore
8.70
自引率
6.00%
发文量
587
审稿时长
1.5 months
期刊介绍:
A multidisciplinary journal focusing on cutting edge research across all fundamental science and technological aspects of catalysis.
Editor-in-chief: Bert Weckhuysen
Impact factor: 5.0
Time to first decision (peer reviewed only): 31 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


