Bioinspired copper-catalysed nitrous oxide reduction with simultaneous N–H or O–H bond oxidation
IF 4.4
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
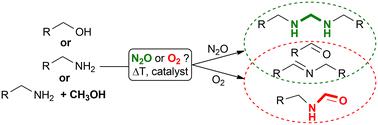
Herein, we report on a bioinspired reduction of nitrous oxide in the presence of hydrogen-donating molecules, which are simultaneously oxidised. The copper-/TEMPO-based molecular catalyst has been previously reported to oxidise, for example, alcohols to aldehydes, diols to lactones, amines to imines, and, more recently, for the N-formylation of amines with methanol using (aerial) oxygen as a terminal oxidant. In this report, we demonstrate that it is possible to decompose nitrous oxide, a natural greenhouse gas and industrial waste gas, at low temperatures. This process simultaneously enables the oxidation of amines to imines and the formation of aminoacetal/aminal through the addition and oxidation of methanol. In this context, the Cu/TEMPO catalyst mimics nitrous oxide reductase (N2OR) and alcohol oxidase (AO) simultaneously. The catalyst is formed in situ from inexpensive and commercially available precursors. Selectivities and yields can be controlled by varying the composition of the substrate mixture and oxidant. This approach is attractive for the synthetic valorisation of organic molecules and utilisation of nitrous oxide, which remains a critical greenhouse gas and a byproduct of large-scale industrial processes, such as fertilizer production. These reactions, facilitated by a robust and affordable catalyst, are easy to carry out, making them highly practical for industrial applications.
生物启发的铜催化氧化亚氮还原,同时进行 N-H 或 O-H 键氧化
在此,我们报告了一种生物还原氧化亚氮的方法,该方法在供氢分子存在的情况下,氧化亚氮同时被氧化。基于铜/TEMPO 的分子催化剂以前曾报道过用于将醇氧化成醛、将二元醇氧化成内酯、将胺氧化成亚胺等,最近还报道过使用(空中)氧作为终端氧化剂,将胺与甲醇进行 N-甲酰化反应。在本报告中,我们展示了在低温下分解一氧化二氮(一种天然温室气体和工业废气)的可能性。这一过程可同时将胺氧化成亚胺,并通过甲醇的加成和氧化形成氨基乙醛/氨基。在这种情况下,Cu/TEMPO 催化剂可同时模拟一氧化二氮还原酶 (N2OR) 和酒精氧化酶 (AO)。该催化剂由廉价的商用前体就地形成。可通过改变底物混合物和氧化剂的组成来控制选择性和产量。这种方法对于有机分子的合成增值和一氧化二氮的利用很有吸引力,一氧化二氮仍然是一种重要的温室气体,也是大规模工业生产过程(如化肥生产)的副产品。这些反应在坚固耐用、经济实惠的催化剂作用下,易于进行,因此在工业应用中非常实用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Catalysis Science & Technology
CHEMISTRY, PHYSICAL-
CiteScore
8.70
自引率
6.00%
发文量
587
审稿时长
1.5 months
期刊介绍:
A multidisciplinary journal focusing on cutting edge research across all fundamental science and technological aspects of catalysis.
Editor-in-chief: Bert Weckhuysen
Impact factor: 5.0
Time to first decision (peer reviewed only): 31 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


