Directed Evolution of Candidatus Methanomethylophilus alvus Pyrrolysyl-tRNA Synthetase for the Genetic Incorporation of Two Different Noncanonical Amino Acids in One Protein
IF 3.8
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
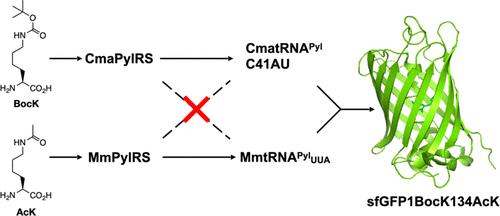
The genetic code expansion technique is a powerful chemical biology tool to install noncanonical amino acids (ncAAs) in proteins. As a key enzyme for this technique, pyrrolysyl-tRNA synthetase (PylRS), coupled with its cognate amber suppressor tRNAPyl, has been engineered for the genetic incorporation of more than 200 ncAAs. Using PylRS clones from different archaeal origins, two ncAAs have also been genetically encoded in one protein. In this work, we show that the C41AU mutant of tRNAPyl from Candidatus Methanomethylophilus alvus (CmatRNAPyl) is catalytically inert toward PylRS from Methanosarcina mazei (MmPylRS) but has weak activity toward PylRS from Ca. M. alvus (CmaPylRS). To improve the catalytic efficiency of CmaPylRS toward CmatRNAPyl-C41AU, we conducted a directed evolution of CMaPylRS by randomizing its coding sequence, followed by the screening of active mutant clones. After three rounds of randomization and screening, we identified 4 mutations, Y16F/N57D/E161G/N182I, that improve the catalytic efficiency of CMaPylRS toward CMatRNAPyl-C41AU. This new clone, named R3–14, coupling with CmatRNAPyl-C41AU to recognize an amber codon, has been successfully used together with an evolved MmPylRS clone, coupling with a mutant M. mazei tRNAPyl to recognize an ochre codon, to genetically incorporate two different ncAAs, Nε-(t-butoxycarbonyl)-lysine and Nε-acetyl-lysine, into one model protein.
嗜甲烷甲酵母菌(Candidatus Methanomethylophilus alvus)吡咯氨酰-tRNA 合成酶的定向进化,在一个蛋白质中遗传性地加入两种不同的非顺式氨基酸
遗传密码扩增技术是一种强大的化学生物学工具,用于在蛋白质中安装非规范氨基酸(ncAA)。作为该技术的关键酶,吡咯乙酰-tRNA 合成酶(PylRS)与其同源的琥珀抑制剂 tRNAPyl 已被设计用于 200 多种 ncAAs 的基因整合。利用来自不同古生菌起源的 PylRS 克隆,还在一个蛋白质中遗传编码了两种 ncAA。在这项工作中,我们发现来自 alvus Methanomethylophilus 的 tRNAPyl 的 C41AU 突变体(CmatRNAPyl)对来自 mazei Methanosarcina 的 PylRS(MmPylRS)具有催化惰性,但对来自 Ca.M.alvus(CmaPylRS)的 PylRS 的活性较弱。为了提高 CmaPylRS 对 CmatRNAPyl-C41AU 的催化效率,我们通过随机化 CMaPylRS 的编码序列对其进行了定向进化,随后筛选出了活性突变克隆。经过三轮随机化和筛选,我们发现 Y16F/N57D/E161G/N182I 等 4 个突变可提高 CMaPylRS 对 CMatRNAPyl-C41AU 的催化效率。这个新克隆被命名为 R3-14,它与 CmatRNAPyl-C41AU 相耦合以识别琥珀色密码子,已被成功地与一个进化的 MmPylRS 克隆(与突变的 M. mazei tRNAPyl 相耦合以识别赭色密码子)一起用于将两种不同的 ncAAs(Nε-(t-丁氧羰基)-赖氨酸和 Nε-acetyl- 赖氨酸)基因整合到一个模型蛋白中。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Bio & Med Chem Au
药物、生物、化学-
CiteScore
4.10
自引率
0.00%
发文量
0
期刊介绍:
ACS Bio & Med Chem Au is a broad scope open access journal which publishes short letters comprehensive articles reviews and perspectives in all aspects of biological and medicinal chemistry. Studies providing fundamental insights or describing novel syntheses as well as clinical or other applications-based work are welcomed.This broad scope includes experimental and theoretical studies on the chemical physical mechanistic and/or structural basis of biological or cell function in all domains of life. It encompasses the fields of chemical biology synthetic biology disease biology cell biology agriculture and food natural products research nucleic acid biology neuroscience structural biology and biophysics.The journal publishes studies that pertain to a broad range of medicinal chemistry including compound design and optimization biological evaluation molecular mechanistic understanding of drug delivery and drug delivery systems imaging agents and pharmacology and translational science of both small and large bioactive molecules. Novel computational cheminformatics and structural studies for the identification (or structure-activity relationship analysis) of bioactive molecules ligands and their targets are also welcome. The journal will consider computational studies applying established computational methods but only in combination with novel and original experimental data (e.g. in cases where new compounds have been designed and tested).Also included in the scope of the journal are articles relating to infectious diseases research on pathogens host-pathogen interactions therapeutics diagnostics vaccines drug-delivery systems and other biomedical technology development pertaining to infectious diseases.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


