Metabolisms of Microlunatus phosphovorus NM-1 Using Glucose, Glutamate, and Aspartate as Carbon Sources for Enhanced Biological Phosphorus Removal
引用次数: 0
Abstract
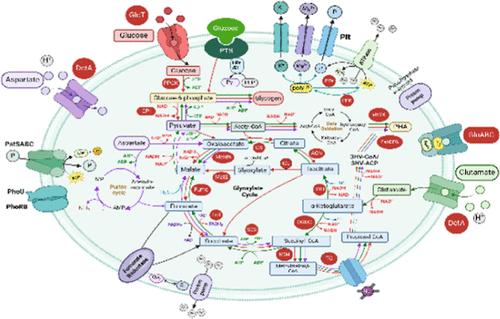
Here, we present the first systematic and comprehensive analysis of the biochemical and transcriptomic characteristics of Microlunatus phosphovorus NM-1 with glucose and amino acids as carbon sources for enhanced biological phosphorus removal (EBPR). Glucose-induced the highest P release rate, followed by aspartate and glutamate. Its anaerobic P release and glucose uptake and aerobic P uptake kinetics exceeded those of Tetrasphaera and Candidatus Accumulibacter (with acetate). Anaerobic glucose uptake and activation were achieved via the phosphoenolpyruvate-dependent phosphotransferase system and bifunctional glucokinases, contributing to its exceptionally high glucose uptake rates. Aspartate and glutamate uptake was driven by proton motive force. Glucose and those amino acids were mainly stored as glycogen. Novel pathways (beta-oxidation and fatty acid biosynthesis) were encoded by NM-1 for polyhydroxyalkanoate generation. Transcriptomic analysis revealed significantly transcribed genes in the glyoxylate cycle in anaerobic glucose metabolism. Glutamate and aspartate were deaminized and routed into the TCA cycle for glycogen and polyhydroxyvalerate generation. Two low-affinity phosphate transporter genes were distinctly transcribed in the anaerobic and aerobic phases, benefiting enhanced P release and uptake. Collectively, this study provides a comprehensive understanding of the glucose and amino acid metabolism of NM-1, benefiting an improved description and modeling of the M. phosphovorus-mediated EBPR process.
以葡萄糖、谷氨酸和天门冬氨酸为碳源加强生物除磷的磷化小水蚤 NM-1 的代谢作用
在此,我们首次系统全面地分析了以葡萄糖和氨基酸为碳源的磷微囊藻(Microlunatus phosphovorus NM-1)在增强生物除磷(EBPR)过程中的生化特征和转录组特征。葡萄糖诱导的磷释放率最高,其次是天冬氨酸和谷氨酸。它的厌氧磷释放和葡萄糖吸收以及需氧磷吸收动力学超过了 Tetrasphaera 和 Candidatus Accumulibacter(使用醋酸盐)。厌氧葡萄糖摄取和活化是通过依赖磷酸烯醇丙酮酸的磷酸转移酶系统和双功能葡萄糖激酶实现的,这也是其葡萄糖摄取率极高的原因。天冬氨酸和谷氨酸的吸收是由质子动力驱动的。葡萄糖和这些氨基酸主要以糖原的形式储存。NM-1 为生成多羟基烷酸编码了新的途径(β-氧化和脂肪酸生物合成)。转录组分析显示,在无氧葡萄糖代谢中,乙醛酸循环中的转录基因明显增加。谷氨酸和天冬氨酸被脱氨基后进入 TCA 循环,用于生成糖原和多羟基戊酸。两个低亲和性磷酸盐转运体基因在无氧和有氧阶段有不同的转录,有利于增强磷酸盐的释放和吸收。总之,本研究提供了对 NM-1 葡萄糖和氨基酸代谢的全面了解,有利于改进对磷脂膜杆菌介导的 EBPR 过程的描述和建模。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


