Phosphate Adsorption from Reclaimed Water via External Cage Expansion on CD-MOF Micro-Interface
引用次数: 0
Abstract
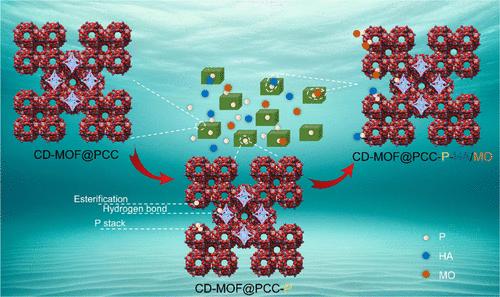
As an essential element for all living organisms, excessive phosphate ranks as a primary contributor to eutrophication in aquatic regions. Coordinative modification of metal–organic frameworks (MOFs) with porous molecular cages can enhance their adsorption selectivity for phosphate. In this study, a novel MOF@porous coordination cages (MOF@PCCs) was developed by modifying PCCs with s-triazine, 2,4,6-tris(2-pyridyl)-s-triazine (tpt), and 2,4,6-tris[4-(pyridin-4-ylmethoxy)phenyl][1,3,5]triazine (tpypt). The results indicated that cyclodextrin-MOF@PCC-L (CD-MOF@PCC-L) prepared with tpypt reached an equilibrium adsorption capacity of 164.5 mg/g within 3 min. Notably, factors such as acidic-neutral pH and low concentrations of anions had negligible effects on phosphate adsorption, while humic acid and methyl orange exhibited a noticeable inhibitory effect on phosphate adsorption. CD-MOF@PCC-L can last for ∼2486 bed volumes before the phosphate concentration in the secondary effluent exceeds the average limit of 0.5 mg/L. Superior phosphorus removal efficiency in adsorption/desorption experiments highlights its potential for effective reclaimed water treatment applications. Comprehensive spectroscopic and computational analyses elucidate the multifaceted phosphate adsorption mechanisms on CD-MOF@PCC-L. Subsequently, the study proposed the application of CD-MOF@PCC after phosphate adsorption for further adsorption removal of humic acid and methyl orange. This study provides innovative insights into microinterface adsorption and an effective strategy for the sequential removal of pollutants via employing phosphate as a bridge.
通过 CD-MOF 微界面上的外笼扩展吸附再生水中的磷酸盐
作为所有生物的必需元素,过量的磷酸盐是造成水域富营养化的主要原因。用多孔分子笼对金属有机框架(MOFs)进行配位修饰可提高其对磷酸盐的吸附选择性。在本研究中,通过用 s-三嗪、2,4,6-三(2-吡啶基)-s-三嗪 (tpt) 和 2,4,6-三[4-(吡啶-4-基甲氧基)苯基][1,3,5]三嗪 (tpypt) 对多孔配位笼进行改性,开发了一种新型 MOF@ 多孔配位笼 (MOF@PCCs)。结果表明,用 tpypt 制备的环糊精-MOF@PCC-L(CD-MOF@PCC-L)在 3 分钟内达到 164.5 mg/g 的平衡吸附容量。值得注意的是,酸性-中性 pH 值和低浓度阴离子等因素对磷酸盐吸附的影响微乎其微,而腐植酸和甲基橙对磷酸盐吸附有明显的抑制作用。CD-MOF@PCC-L 在二级出水磷酸盐浓度超过 0.5 毫克/升的平均限值之前,可持续吸附 2486 个床层体积。在吸附/解吸实验中,其卓越的除磷效率凸显了其在有效再生水处理应用中的潜力。综合光谱和计算分析阐明了 CD-MOF@PCC-L 对磷酸盐的多元吸附机理。随后,研究提出了 CD-MOF@PCC 在吸附磷酸盐后进一步吸附去除腐殖酸和甲基橙的应用。这项研究为微界面吸附提供了创新见解,并为以磷酸盐为桥梁连续去除污染物提供了有效策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


