HN1-mediated activation of lipogenesis through Akt-SREBP signaling promotes hepatocellular carcinoma cell proliferation and metastasis
IF 4.8
3区 医学
Q1 BIOTECHNOLOGY & APPLIED MICROBIOLOGY
引用次数: 0
Abstract
Hepatocellular carcinoma (HCC) is the second leading cause of cancer-related deaths worldwide, with more than 800,000 deaths each year, and its 5-year survival rate is less than 12%. The role of the HN1 gene in HCC has remained elusive, despite its upregulation in various cancer types. In our investigation, we identified HN1’s heightened expression in HCC tissues, which, upon overexpression, fosters cell proliferation, migration, and invasion, unveiling its role as an oncogene in HCC. In addition, silencing HN1 diminished the viability and metastasis of HCC cells, whereas HN1 overexpression stimulated their growth and invasion. Gene expression profiling revealed HN1 silencing downregulated 379 genes and upregulated 130 genes, and suppressive proteins associated with the lipogenic signaling pathway networks. Notably, suppressing HN1 markedly decreased the expression levels of SREBP1 and SREBP2, whereas elevating HN1 had the converse effect. This dual modulation of HN1 affected lipid formation, hindering it upon HN1 silencing and promoting it upon HN1 overexpression. Moreover, HN1 triggers the Akt pathway, fostering tumorigenesis via SREBP1-mediated lipogenesis and silencing HN1 effectively curbed HCC tumor growth in mouse xenograft models by deactivating SREBP-1, emphasizing the potential of HN1 as a therapeutic target, impacting both external and internal factors, it holds promise as an effective therapeutic strategy for HCC.
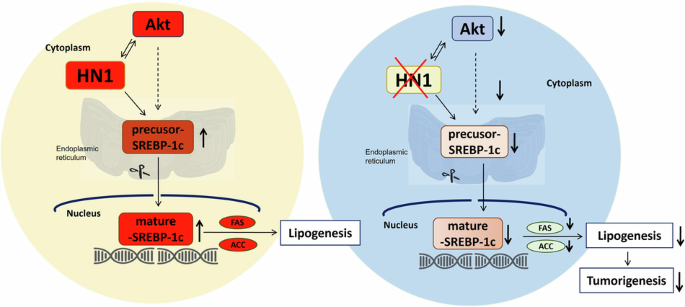
HN1 通过 Akt-SREBP 信号介导激活脂肪生成,促进肝癌细胞增殖和转移
肝细胞癌(HCC)是全球癌症相关死亡的第二大原因,每年有 80 多万人死于此病,其 5 年生存率不到 12%。尽管 HN1 基因在多种癌症类型中上调,但其在 HCC 中的作用仍然难以捉摸。在我们的研究中,我们发现了 HN1 在 HCC 组织中的高表达,过表达会促进细胞增殖、迁移和侵袭,从而揭示了它在 HCC 中的癌基因作用。此外,沉默 HN1 会降低 HCC 细胞的活力和转移性,而过表达 HN1 则会刺激其生长和侵袭。基因表达谱分析显示,沉默HN1会下调379个基因,上调130个基因,并抑制与脂肪生成信号通路网络相关的蛋白。值得注意的是,抑制 HN1 会显著降低 SREBP1 和 SREBP2 的表达水平,而提高 HN1 则会产生相反的效果。HN1 的这种双重调节作用影响了脂质的形成,抑制 HN1 会阻碍脂质的形成,而过量表达 HN1 则会促进脂质的形成。此外,HN1还能触发Akt通路,通过SREBP1介导的脂肪生成促进肿瘤发生,而沉默HN1能通过使SREBP-1失活有效抑制HCC肿瘤在小鼠异种移植模型中的生长。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cancer gene therapy
医学-生物工程与应用微生物
CiteScore
10.20
自引率
0.00%
发文量
150
审稿时长
4-8 weeks
期刊介绍:
Cancer Gene Therapy is the essential gene and cellular therapy resource for cancer researchers and clinicians, keeping readers up to date with the latest developments in gene and cellular therapies for cancer. The journal publishes original laboratory and clinical research papers, case reports and review articles. Publication topics include RNAi approaches, drug resistance, hematopoietic progenitor cell gene transfer, cancer stem cells, cellular therapies, homologous recombination, ribozyme technology, antisense technology, tumor immunotherapy and tumor suppressors, translational research, cancer therapy, gene delivery systems (viral and non-viral), anti-gene therapy (antisense, siRNA & ribozymes), apoptosis; mechanisms and therapies, vaccine development, immunology and immunotherapy, DNA synthesis and repair.
Cancer Gene Therapy publishes the results of laboratory investigations, preclinical studies, and clinical trials in the field of gene transfer/gene therapy and cellular therapies as applied to cancer research. Types of articles published include original research articles; case reports; brief communications; review articles in the main fields of drug resistance/sensitivity, gene therapy, cellular therapy, tumor suppressor and anti-oncogene therapy, cytokine/tumor immunotherapy, etc.; industry perspectives; and letters to the editor.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


