Mass Spectrometric Study of Evaporation of Hydroxyapatite at Temperatures up to 2200 K
IF 0.7
4区 化学
Q4 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
The composition of the gas phase was identified for the first time up to a temperature of 2200 K by the Knudsen effusion mass spectrometric method, and the partial pressures of the vapor species during the evaporation of hydroxyapatite were determined. Ca10(PO4)6(OH)2 was evaporated from a Knudsen effusion cell made of tungsten. In the temperature range 1200–1300 K, Ca10(PO4)6(OH)2 undergoes dehydration, forming Ca3P2O8, which evaporates in the form of PO, atomic calcium, and oxygen when the temperature further increases to 1750–2200 K.

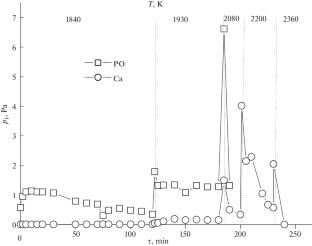
温度高达 2200 K 时羟基磷灰石蒸发的质谱研究
摘要 利用克努森渗流质谱法首次鉴定了温度高达 2200 K 的气相组成,并测定了羟基磷灰石蒸发过程中气态物质的分压。Ca10(PO4)6(OH)2是从钨制成的克努森渗流池中蒸发出来的。在 1200-1300 K 的温度范围内,Ca10(PO4)6(OH)2 发生脱水,形成 Ca3P2O8,当温度进一步升高到 1750-2200 K 时,Ca3P2O8 以 PO、原子钙和氧的形式蒸发。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
1.20
自引率
14.30%
发文量
376
审稿时长
5.1 months
期刊介绍:
Russian Journal of Physical Chemistry A. Focus on Chemistry (Zhurnal Fizicheskoi Khimii), founded in 1930, offers a comprehensive review of theoretical and experimental research from the Russian Academy of Sciences, leading research and academic centers from Russia and from all over the world.
Articles are devoted to chemical thermodynamics and thermochemistry, biophysical chemistry, photochemistry and magnetochemistry, materials structure, quantum chemistry, physical chemistry of nanomaterials and solutions, surface phenomena and adsorption, and methods and techniques of physicochemical studies.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


