Production of recombinant adeno-associated virus 5 using a novel self-attenuating adenovirus production platform
IF 4.6
2区 医学
Q2 MEDICINE, RESEARCH & EXPERIMENTAL
Molecular Therapy-Methods & Clinical Development
Pub Date : 2024-08-14
DOI:10.1016/j.omtm.2024.101320
引用次数: 0
Abstract
Recombinant adeno-associated virus (rAAV) has become a prominent vector for clinical use. Despite an increase in successful clinical outcomes, the amount of high-quality rAAVs required for clinical trials and eventual commercial demand is difficult to produce, especially for genetic diseases that are prevalent or require high doses. Many groups are focused on establishing production processes that can produce sufficient rAAV while maintaining potency and quality. Our group used a novel production platform to increase our yield of rAAV5. This production platform uses tetracycline-enabled self-silencing adenovirus (TESSA) to deliver the wild-type AAV replication and capsid genes alongside the adenovirus helper genes necessary for production. Here, we describe our efforts to evaluate the TESSA platform in house. We conducted numerous experiments to determine the optimal conditions for producing rAAV5 from the TESSA production system. We then produced rAAV5 from the TESSA system to compare against rAAV5 produced from triple transfection. Ultimately, we generated data that showed that the vector genome yield of rAAV5 produced with TESSA was >20-fold higher than rAAV5 produced with triple transfection. Additionally, our data show that quality as well as potency in mice of rAAV5 produced with the TESSA system and by triple transfection are equivalent.
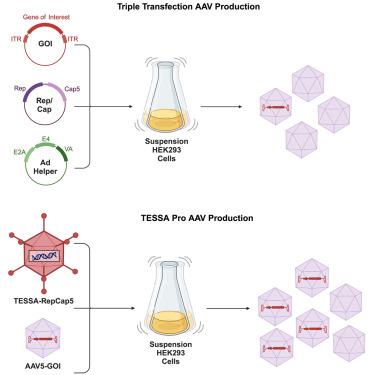
利用新型自增强腺病毒生产平台生产重组腺相关病毒 5
重组腺相关病毒(rAAV)已成为临床使用的主要载体。尽管成功的临床结果越来越多,但临床试验和最终商业需求所需的高质量 rAAV 却很难生产,特别是对于流行性或需要高剂量的遗传疾病。许多研究小组都在致力于建立既能生产足够数量的 rAAV,又能保持效力和质量的生产工艺。我们小组使用了一种新型生产平台来提高 rAAV5 的产量。该生产平台使用四环素自沉默腺病毒(TESSA)来传递野生型 AAV 复制和囊膜基因以及生产所需的腺病毒辅助基因。在此,我们介绍了我们为评估 TESSA 平台所做的努力。我们进行了大量实验,以确定用 TESSA 生产系统生产 rAAV5 的最佳条件。然后,我们将 TESSA 系统生产的 rAAV5 与三重转染生产的 rAAV5 进行比较。最终,我们得出的数据显示,用 TESSA 生产的 rAAV5 的载体基因组产量比用三重转染生产的 rAAV5 高出 20 倍以上。此外,我们的数据还表明,用 TESSA 系统和三重转染法生产的 rAAV5 在小鼠体内的质量和效力相当。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Molecular Therapy-Methods & Clinical Development
Biochemistry, Genetics and Molecular Biology-Molecular Biology
CiteScore
9.90
自引率
4.30%
发文量
163
审稿时长
12 weeks
期刊介绍:
The aim of Molecular Therapy—Methods & Clinical Development is to build upon the success of Molecular Therapy in publishing important peer-reviewed methods and procedures, as well as translational advances in the broad array of fields under the molecular therapy umbrella.
Topics of particular interest within the journal''s scope include:
Gene vector engineering and production,
Methods for targeted genome editing and engineering,
Methods and technology development for cell reprogramming and directed differentiation of pluripotent cells,
Methods for gene and cell vector delivery,
Development of biomaterials and nanoparticles for applications in gene and cell therapy and regenerative medicine,
Analysis of gene and cell vector biodistribution and tracking,
Pharmacology/toxicology studies of new and next-generation vectors,
Methods for cell isolation, engineering, culture, expansion, and transplantation,
Cell processing, storage, and banking for therapeutic application,
Preclinical and QC/QA assay development,
Translational and clinical scale-up and Good Manufacturing procedures and process development,
Clinical protocol development,
Computational and bioinformatic methods for analysis, modeling, or visualization of biological data,
Negotiating the regulatory approval process and obtaining such approval for clinical trials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


