Lipid nanoparticle encapsulation of a Delta spike-CD40L DNA vaccine improves effectiveness against Omicron challenge in Syrian hamsters
IF 4.7
2区 医学
Q2 MEDICINE, RESEARCH & EXPERIMENTAL
Molecular Therapy-Methods & Clinical Development
Pub Date : 2024-08-19
DOI:10.1016/j.omtm.2024.101325
引用次数: 0
Abstract
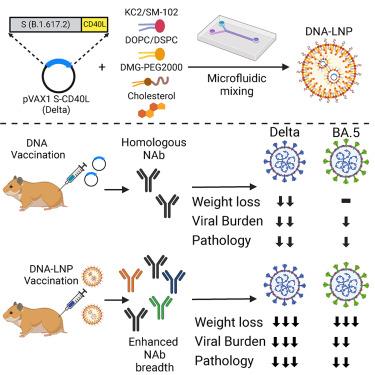
The effectiveness of mRNA vaccines largely depends on their lipid nanoparticle (LNP) component. Herein, we investigate the effectiveness of DLin-KC2-DMA (KC2) and SM-102-based LNPs for the intramuscular delivery of a plasmid encoding B.1.617.2 (Delta) spike fused with CD40 ligand. LNP encapsulation of this CD40L-adjuvanted DNA vaccine with either LNP formulation drastically enhanced antibody responses, enabling neutralization of heterologous Omicron variants. The DNA-LNP formulations provided excellent protection from homologous challenge, reducing viral replication, and preventing histopathological changes in the pulmonary tissues. Moreover, the DNA-LNP vaccines maintained a high level of protection against heterologous Omicron BA.5 challenge despite a reduced neutralizing response. In addition, we observed that DNA-LNP vaccination led to the pulmonary downregulation of interferon signaling, interleukin-12 signaling, and macrophage response pathways following SARS-CoV-2 challenge, shedding some light on the mechanisms underlying the prevention of pulmonary injury. These results highlight the potential combination of molecular adjuvants with LNP-based vaccine delivery to induce greater and broader immune responses capable of preventing inflammatory damage and protecting against emerging variants. These findings could be informative for the future design of both DNA and mRNA vaccines.
用脂质纳米颗粒封装 Delta spike-CD40L DNA 疫苗可提高叙利亚仓鼠抵抗 Omicron 挑战的效力
mRNA 疫苗的有效性在很大程度上取决于其脂质纳米颗粒(LNP)成分。在此,我们研究了基于 DLin-KC2-DMA (KC2) 和 SM-102 的 LNP 肌肉内递送编码与 CD40 配体融合的 B.1.617.2 (Delta) 穗状病毒质粒的有效性。用任何一种 LNP 制剂封装这种 CD40L 佐剂 DNA 疫苗都能显著增强抗体反应,从而中和异源 Omicron 变种。DNA-LNP 制剂能很好地抵御同源挑战,减少病毒复制,防止肺组织发生组织病理学变化。此外,DNA-LNP 疫苗在面对异源 Omicron BA.5 挑战时也能保持较高的保护能力,尽管中和反应有所降低。此外,我们还观察到,接种 DNA-LNP 疫苗后,干扰素信号、白细胞介素-12 信号和巨噬细胞反应途径在 SARS-CoV-2 挑战后的肺部下调,从而揭示了预防肺损伤的机制。这些结果凸显了分子佐剂与基于 LNP 的疫苗递送相结合的潜力,可诱导更强、更广泛的免疫反应,从而预防炎症损伤并抵御新出现的变种。这些发现可能对未来 DNA 和 mRNA 疫苗的设计具有参考价值。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Molecular Therapy-Methods & Clinical Development
Biochemistry, Genetics and Molecular Biology-Molecular Biology
CiteScore
9.90
自引率
4.30%
发文量
163
审稿时长
12 weeks
期刊介绍:
The aim of Molecular Therapy—Methods & Clinical Development is to build upon the success of Molecular Therapy in publishing important peer-reviewed methods and procedures, as well as translational advances in the broad array of fields under the molecular therapy umbrella.
Topics of particular interest within the journal''s scope include:
Gene vector engineering and production,
Methods for targeted genome editing and engineering,
Methods and technology development for cell reprogramming and directed differentiation of pluripotent cells,
Methods for gene and cell vector delivery,
Development of biomaterials and nanoparticles for applications in gene and cell therapy and regenerative medicine,
Analysis of gene and cell vector biodistribution and tracking,
Pharmacology/toxicology studies of new and next-generation vectors,
Methods for cell isolation, engineering, culture, expansion, and transplantation,
Cell processing, storage, and banking for therapeutic application,
Preclinical and QC/QA assay development,
Translational and clinical scale-up and Good Manufacturing procedures and process development,
Clinical protocol development,
Computational and bioinformatic methods for analysis, modeling, or visualization of biological data,
Negotiating the regulatory approval process and obtaining such approval for clinical trials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


