Unlocking DOE potential by selecting the most appropriate design for rAAV optimization
IF 4.6
2区 医学
Q2 MEDICINE, RESEARCH & EXPERIMENTAL
Molecular Therapy-Methods & Clinical Development
Pub Date : 2024-08-26
DOI:10.1016/j.omtm.2024.101329
引用次数: 0
Abstract
Producing recombinant adeno-associated virus (rAAV) for gene therapy via triple transfection is an intricate process involving many cellular interactions. Each of the different elements encoded in the three required plasmids—pHelper, pRepCap, and pGOI—plays a distinct role, affecting different cellular pathways when producing rAAVs. The required expression balance emphasizes the critical need to fine-tune the concentration of all these different elements. The use of design of experiments (DOE) to find optimal ratios is a powerful method to streamline the process. However, the choice of the DOE method and design construction is crucial to avoid misleading results. In this work, we examined and compared four distinct DOE approaches: rotatable central composite design (RCCD), Box-Behnken design (BBD), face-centered central composite design (FCCD), and mixture design (MD). We compared the abilities of the different models to predict optimal ratios and interactions among the plasmids and the transfection reagent. Our findings revealed that blocking is essential to reduce the variability caused by uncontrolled random effects and that MD coupled with FCCD outperformed all other approaches, improving volumetric productivity 109-fold. These outcomes underscore the importance of selecting a model that can effectively account for the biological context, ultimately yielding superior results in optimizing rAAV production.
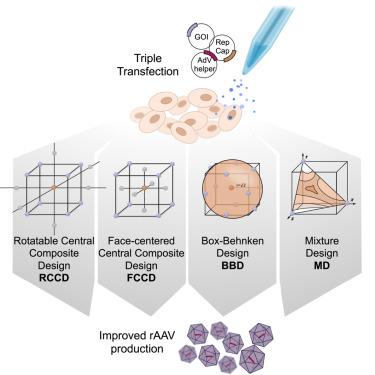
为 rAAV 优化选择最合适的设计,释放 DOE 潜能
通过三重转染生产用于基因治疗的重组腺相关病毒(rAAV)是一个涉及多种细胞相互作用的复杂过程。所需的三个质粒--pHelper、pRepCap 和 pGOI--中编码的每个不同元件都发挥着不同的作用,在生产 rAAV 时影响不同的细胞通路。所需的表达平衡强调了微调所有这些不同元素浓度的关键必要性。使用实验设计(DOE)寻找最佳配比是简化这一过程的有效方法。然而,选择 DOE 方法和设计结构对于避免误导结果至关重要。在这项工作中,我们研究并比较了四种不同的 DOE 方法:可旋转中心复合设计 (RCCD)、方框-贝肯设计 (BBD)、面心中心复合设计 (FCCD) 和混合设计 (MD)。我们比较了不同模型预测质粒和转染试剂之间最佳比例和相互作用的能力。我们的研究结果表明,阻断对于减少不受控制的随机效应造成的变异性至关重要,而 MD 与 FCCD 的结合优于所有其他方法,可将体积生产率提高 109 倍。这些结果凸显了选择一个能有效解释生物背景的模型的重要性,最终将在优化 rAAV 生产方面产生卓越的结果。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Molecular Therapy-Methods & Clinical Development
Biochemistry, Genetics and Molecular Biology-Molecular Biology
CiteScore
9.90
自引率
4.30%
发文量
163
审稿时长
12 weeks
期刊介绍:
The aim of Molecular Therapy—Methods & Clinical Development is to build upon the success of Molecular Therapy in publishing important peer-reviewed methods and procedures, as well as translational advances in the broad array of fields under the molecular therapy umbrella.
Topics of particular interest within the journal''s scope include:
Gene vector engineering and production,
Methods for targeted genome editing and engineering,
Methods and technology development for cell reprogramming and directed differentiation of pluripotent cells,
Methods for gene and cell vector delivery,
Development of biomaterials and nanoparticles for applications in gene and cell therapy and regenerative medicine,
Analysis of gene and cell vector biodistribution and tracking,
Pharmacology/toxicology studies of new and next-generation vectors,
Methods for cell isolation, engineering, culture, expansion, and transplantation,
Cell processing, storage, and banking for therapeutic application,
Preclinical and QC/QA assay development,
Translational and clinical scale-up and Good Manufacturing procedures and process development,
Clinical protocol development,
Computational and bioinformatic methods for analysis, modeling, or visualization of biological data,
Negotiating the regulatory approval process and obtaining such approval for clinical trials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


