The Antiproteinopathy, Antioxidant, and Antiapoptotic Effects of Methylene Blue and 4-Phenylbutyric Acid Alone, and in Combination on Familial Alzheimer’s Disease PSEN1 I416T Cholinergic-Like Neurons
IF 4.1
3区 医学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
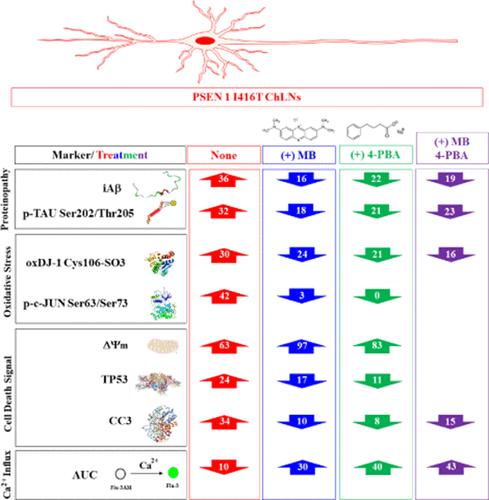
Familial Alzheimer’s disease (FAD) is a chronic neurological condition that progresses over time. Currently, lacking a viable treatment, the use of multitarget medication combinations has generated interest as a potential FAD therapy approach. In this study, we examined the effects of 4-phenylbutyric acid (4-PBA) and methylene blue (MB) either separately or in combination on PSEN1 I416T cholinergic-like neuron cells (ChLNs), which serve as a model for FAD. We found that MB was significantly efficient at reducing the accumulation of intracellular Aβ, phosphorylation of TAU Ser202/Thr205, and increasing Δψm, whereas 4-PBA was significantly efficient at diminishing oxidation of DJ-1Cys106-SH, expression of TP53, and increasing ACh-induced Ca2+ influx. Both agents were equally effective at blunting phosphorylated c-JUN at Ser63/Ser73 and activating caspase 3 (CASP3) into cleaved caspase 3 (CC3) on mutant cells. Combination of MB and 4-PBA at middle (0.1, 1) concentration significantly reduced iAβ, p-TAU, and oxDJ-1 and augmented the ACh-induced Ca2+ influx compared to combined agents at low (0.05, 0.5) or high (0.5, 5) concentration. However, combined MB and 4-PBA were efficient only at dropping DJ-1Cys106-SO3 and increasing ACh-induced Ca2+ inward in mutant ChLNs. Our data show that the reagents MB and 4-PBA alone possess more than one action (e.g., antiamyloid, antioxidant, anti-TAU, antiapoptotic, and ACh-induced Ca2+ influx enhancers), that in combination might cancel or diminish each other. Together, these results strongly argue that MB and 4-PBA might protect PSEN1 I416T ChLNs from Aβ-induced toxicity by working intracellularly as anti-Aβ and anti-Tau agents, improving Δψm and cell survival, and extracellularly, by increasing ACh-induced Ca2+ ion influx. MB and 4-PBA are promising drugs with potential for repurposing in familial AD.
亚甲基蓝和 4-苯基丁酸单独或联合使用对家族性阿尔茨海默病 PSEN1 I416T 胆碱能样神经元的抗蛋白病、抗氧化和抗凋亡作用
家族性阿尔茨海默病(FAD)是一种慢性神经系统疾病,会随着时间的推移而恶化。目前,由于缺乏可行的治疗方法,使用多靶点药物组合作为一种潜在的 FAD 治疗方法引起了人们的兴趣。在这项研究中,我们研究了 4-苯基丁酸(4-PBA)和亚甲基蓝(MB)单独或联合使用对 PSEN1 I416T 胆碱能样神经元细胞(ChLNs)的影响,该细胞是 FAD 的模型。我们发现,甲基溴在减少细胞内 Aβ 的积累、TAU Ser202/Thr205 的磷酸化和增加 Δψm 方面有明显的效果,而 4-PBA 在减少 DJ-1Cys106-SH 的氧化、TP53 的表达和增加 ACh 诱导的 Ca2+ 流入方面有明显的效果。这两种制剂在抑制突变细胞上磷酸化 c-JUN 的 Ser63/Ser73 和激活 caspase 3(CASP3)转化为裂解 caspase 3(CC3)方面同样有效。与低浓度(0.05,0.5)或高浓度(0.5,5)的联合用药相比,中浓度(0.1,1)的 MB 和 4-PBA 联合用药可显著降低 iAβ、p-TAU 和 oxDJ-1,并增强 ACh 诱导的 Ca2+ 流入。然而,MB 和 4-PBA 复方制剂仅能有效降低突变 ChLN 中的 DJ-1Cys106-SO3 和增加 ACh 诱导的 Ca2+内流。我们的数据表明,MB 和 4-PBA 试剂本身具有一种以上的作用(如抗淀粉样蛋白、抗氧化、抗TAU、抗细胞凋亡和 ACh 诱导的 Ca2+ 流入增强剂),两者结合使用可能会相互抵消或削弱。总之,这些结果有力地证明了 MB 和 4-PBA 可保护 PSEN1 I416T ChLNs 免受 Aβ 诱导的毒性,它们在细胞内起抗 Aβ 和抗 Tau 的作用,改善Δψm 和细胞存活,在细胞外起增加 ACh 诱导的 Ca2+ 离子流入的作用。MB和4-PBA是很有前途的药物,有可能用于家族性AD的再用途。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Chemical Neuroscience
BIOCHEMISTRY & MOLECULAR BIOLOGY-CHEMISTRY, MEDICINAL
CiteScore
9.20
自引率
4.00%
发文量
323
审稿时长
1 months
期刊介绍:
ACS Chemical Neuroscience publishes high-quality research articles and reviews that showcase chemical, quantitative biological, biophysical and bioengineering approaches to the understanding of the nervous system and to the development of new treatments for neurological disorders. Research in the journal focuses on aspects of chemical neurobiology and bio-neurochemistry such as the following:
Neurotransmitters and receptors
Neuropharmaceuticals and therapeutics
Neural development—Plasticity, and degeneration
Chemical, physical, and computational methods in neuroscience
Neuronal diseases—basis, detection, and treatment
Mechanism of aging, learning, memory and behavior
Pain and sensory processing
Neurotoxins
Neuroscience-inspired bioengineering
Development of methods in chemical neurobiology
Neuroimaging agents and technologies
Animal models for central nervous system diseases
Behavioral research
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


