A Mass Spectrometry Experiment on the Degrees of Freedom Effect
IF 2.9
3区 教育学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
An experiment is described that allows students to investigate the physical chemistry involved in unimolecular fragmentation and to explain their experimental results using Rice–Ramsperger–Kassel (RRK) theory. For the experiment, students prepare a solution of a series of homologous analytes and collect mass spectra of those analytes using GC-MS. All the analytes undergo the same three-step fragmentation in the mass spectrometer. The relative peak intensities from the ions in the fragmentation scheme are plotted as a function of the vibrational degrees of freedom of the molecular ions’ R group. Trends in these plots can be explained using concepts from RRK theory and highlight the “degrees of freedom effect” in mass spectrometry. The experiment is suitable for a variety of upper-level, undergraduate chemistry laboratories.
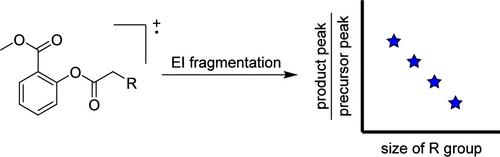
关于自由度效应的质谱实验
本实验让学生探究单分子破碎所涉及的物理化学原理,并用赖斯-拉姆伯格-卡塞尔(RRK)理论解释实验结果。在实验中,学生制备一系列同源分析物的溶液,并使用气相色谱-质谱仪收集这些分析物的质谱。所有分析物在质谱仪中都经历了相同的三步破碎。碎裂方案中离子的相对峰强度是分子离子 R 基团振动自由度的函数。这些图中的趋势可以用 RRK 理论的概念来解释,并突出了质谱分析中的 "自由度效应"。该实验适用于各种高年级本科生化学实验室。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Chemical Education
化学-化学综合
CiteScore
5.60
自引率
50.00%
发文量
465
审稿时长
6.5 months
期刊介绍:
The Journal of Chemical Education is the official journal of the Division of Chemical Education of the American Chemical Society, co-published with the American Chemical Society Publications Division. Launched in 1924, the Journal of Chemical Education is the world’s premier chemical education journal. The Journal publishes peer-reviewed articles and related information as a resource to those in the field of chemical education and to those institutions that serve them. JCE typically addresses chemical content, activities, laboratory experiments, instructional methods, and pedagogies. The Journal serves as a means of communication among people across the world who are interested in the teaching and learning of chemistry. This includes instructors of chemistry from middle school through graduate school, professional staff who support these teaching activities, as well as some scientists in commerce, industry, and government.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


