Orthogonal Linking of Gemcitabine by Acyclovir and Bortezomib: A Rational Chemistry Laboratory Introduction of Both Noncovalent and Covalent Dynamics
IF 2.9
3区 教育学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
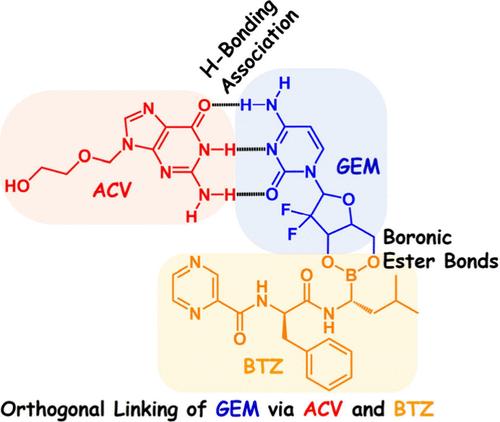
Constitutional dynamic chemistry (CDC), as defined by Lehn ( Chem. Soc. Rev. 2007, 36, 151−160), covers both supramolecular science and dynamic covalent chemistry to process components recombination toward the chemistry of complex matter. While CDC has increasingly guided active research decisions during the past decades, undergraduate chemistry curricula do not reflect the prevalence of such concepts and knowledge; this article seeks to introduce such ideas to laboratory practice. Incorporation of CDC and its characterization techniques, such as 1H NMR and mass spectrometry (MS), into chemistry education is warranted to support CDC-relevant learning experiences, understanding of spectroscopy instrumentation, and student motivation to pursue a professional career. Herein, we develop an experimental design by combining supramolecular forces, i.e., H-bonding interaction, and dynamic covalent linkage, i.e., borate ester bond, toward orthogonal linking of the chemotherapeutic agent gemcitabine (GEM) by the antiviral drug acyclovir (ACV) and proteasome inhibitor bortezomib (BTZ). Such design enables a “double dynamic” process, which leverages both noncovalent and covalent dynamics. Moreover, this laboratory program outlines how NMR and MS technologies are employed to characterize H-bonding association and boronic ester bonds. Such a lab experiment provides the prospect for instructors to illustrate to undergraduates with a basic background in chemistry and medical science the state-of-the-art H-bonding interaction and boronic ester bond, modern characterization instruments, and broad impacts of CDC.
阿昔洛韦和硼替佐米对吉西他滨的正交连接:非共价和共价动力学的理性化学实验室介绍
莱恩(Chem. Soc. Rev. 2007, 36, 151-160)定义的 "立宪动态化学(CDC)"涵盖了超分子科学和动态共价化学,以处理复杂物质化学的组分重组。在过去几十年中,CDC 越来越多地指导着积极的研究决策,但本科生化学课程并未反映出此类概念和知识的普及;本文旨在将此类观点引入实验室实践。将 CDC 及其表征技术(如 1H NMR 和质谱法 (MS))纳入化学教育是有必要的,以支持与 CDC 相关的学习体验、对光谱仪器的理解以及学生追求专业职业的动力。在此,我们结合超分子力(即 H 键相互作用)和动态共价连接(即硼酸酯键),开发了一种实验设计,以实现抗病毒药物阿昔洛韦(ACV)和蛋白酶体抑制剂硼替佐米(BTZ)正交连接化疗药物吉西他滨(GEM)。这种设计实现了 "双动态 "过程,既利用了非共价动态,又利用了共价动态。此外,该实验室计划还概述了如何利用 NMR 和 MS 技术来表征 H 键关联和硼酸酯键。这样的实验为具有化学和医学基础背景的本科生提供了机会,让他们了解最先进的 H 键相互作用和硼酸酯键、现代表征仪器以及 CDC 的广泛影响。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Chemical Education
化学-化学综合
CiteScore
5.60
自引率
50.00%
发文量
465
审稿时长
6.5 months
期刊介绍:
The Journal of Chemical Education is the official journal of the Division of Chemical Education of the American Chemical Society, co-published with the American Chemical Society Publications Division. Launched in 1924, the Journal of Chemical Education is the world’s premier chemical education journal. The Journal publishes peer-reviewed articles and related information as a resource to those in the field of chemical education and to those institutions that serve them. JCE typically addresses chemical content, activities, laboratory experiments, instructional methods, and pedagogies. The Journal serves as a means of communication among people across the world who are interested in the teaching and learning of chemistry. This includes instructors of chemistry from middle school through graduate school, professional staff who support these teaching activities, as well as some scientists in commerce, industry, and government.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


