Bismuth oxide nanoparticles as a promising adsorbent for removal of quetiapine: synthesis, characterization, and application
Abstract
BACKGROUND
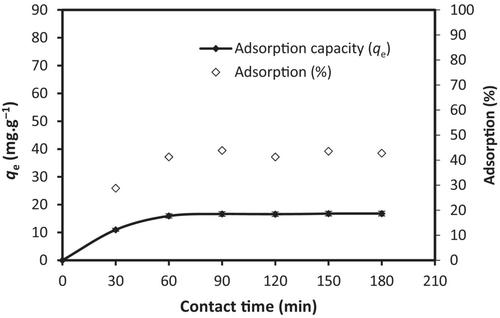
Within the scope of this study, bismuth oxide (Bi2O3) nanoparticles have been synthesized by a simple method, and the usability of these nanoparticles as adsorbents has been investigated. For this purpose, Bi2O3 nanoparticles were synthesized by a chemical method. These particles were characterized by various methods such as Fourier transform infrared spectroscopy, Brunauer–Emmett–Teller surface area, scanning electron microscopy, and X-ray diffraction. The nature of the synthesized nanoparticles was confirmed by characterization results and the synthesized particles were found to be nanoscale. As a result of the characterization, the average particle diameters and surface areas were found to be 22.24, 32.24 and 49.98 nm, and 5.95, 3.54 and 0.75 m2 g−1 for different calcination temperatures of 105, 250 and 600 °C, respectively. Adsorption parameters such as initial quetiapine concentration, bismuth oxide nanoparticle dosage, temperature, equilibrium contact time, and pH were also studied. Moreover, kinetic, isotherm, and thermodynamics modeling of adsorption have been performed to account for the adsorption mechanism of quetiapine by Bi2O3 nanoparticles.
RESULTS
The thermodynamic study has specified that adsorption has been spontaneous and exothermic. The kinetic study has pointed out that a pseudo-second-order model (R2 = 0.9853) has been favorable to the data. Furthermore, a Freundlich isotherm model (R2 = 0.8258) has been better fitted to the experimental adsorption results. The maximum adsorption capacity and percentage of adsorption values were 27.38 mg g−1 and 76.81%, respectively.
CONCLUSION
The outcomes demonstrate that synthesized Bi2O3 nanoparticle is an influential adsorbent for removing quetiapine. Also, the obtained results enabled us to estimate the possibility of using Bi2O3 nanoparticles to remove active pharmaceutical ingredients by adsorption. © 2024 The Author(s). Journal of Chemical Technology and Biotechnology published by John Wiley & Sons Ltd on behalf of Society of Chemical Industry (SCI).

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


