Agar-Activated Carbon Cathode with Optimized Redox Electrolyte for High-Energy and Stable Aqueous Zinc Hybrid Battery–Capacitor
IF 5.5
3区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
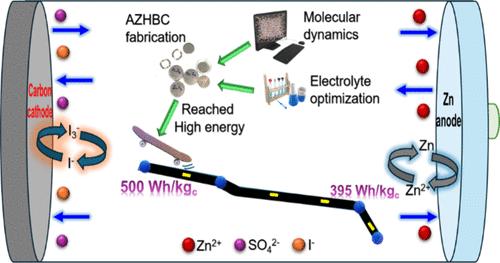
Recently, aqueous zinc hybrid battery–capacitors (AZHBCs) have received significant attention owing to advantages such as low cost, high safety, high power, and a long cycle life. However, the limited energy density of the current AZHBCs should be further improved by introducing cathode materials and optimized electrolytes to realize their large-scale applications. In this study, agar biopolymer-based activated carbon optimized at 750 °C (AAC-750) with large specific surface area, an optimized aqueous solution of ZnSO4 + KI, and zinc metal were employed as the cathode material, redox electrolyte, and anode, respectively. An optimal AZHBC configuration, consisting of AAC-750//2 M ZnSO4 + 0.3 M KI//Zn, exhibited an outstanding high capacity of 413 mAh gc–1, a remarkable energy density (∼508 Wh kgc–1) that breached the milestone of 500 Wh kgc–1 at 0.1 A gc–1 in the voltage range of 0.2–1.8 V, and an excellent cyclic stability with a capacity retention of 97% over 10 000 cycles at 10 A g–1. In terms of the mechanism, this remarkable performance can be ascribed to the polyiodide redox reaction (3I–/I3–, 2I–/I2, and 2I3–/3I2), the reversible ion adsorption of SO42–/I– at the cathode, and the reversible electrodeposition of Zn2+ at the anode, respectively. In addition, theoretical analyses were carried out to understand the molecular dynamics of various aqueous ZnSO4 + KI electrolyte systems and the adsorption process of polyiodide ions. The proposed strategy provides a way to design high-energy and stable AZHBCs with an appropriate electrolyte system and a biopolymer-derived carbon cathode.
含优化氧化还原电解质的琼脂活性炭阴极用于高能量和稳定的锌水混合电池-电容器
最近,锌水混合电池电容器(AZHBCs)因其低成本、高安全性、高功率和长循环寿命等优点而受到广泛关注。然而,目前 AZHBCs 的能量密度有限,应通过引入阴极材料和优化电解质进一步提高其能量密度,以实现其大规模应用。在本研究中,采用了比表面积大、温度为 750 °C 的琼脂生物聚合物基活性炭(AAC-750)、优化的 ZnSO4 + KI 水溶液和金属锌分别作为阴极材料、氧化还原电解质和阳极。由 AAC-750//2 M ZnSO4 + 0.3 M KI//Zn 组成的最佳 AZHBC 配置显示出 413 mAh gc-1 的出色高容量,在 0.2-1.8 V 的电压范围内,0.1 A gc-1 的能量密度(∼508 Wh kgc-1)突破了 500 Wh kgc-1 的里程碑。从机理上看,这一卓越性能可分别归因于聚碘氧化还原反应(3I-/I3-、2I-/I2 和 2I3-/3I2)、阴极对 SO42-/I- 的可逆离子吸附以及阳极对 Zn2+ 的可逆电沉积。此外,还进行了理论分析,以了解各种 ZnSO4 + KI 水电解质体系的分子动力学和多碘离子的吸附过程。所提出的策略为利用适当的电解质体系和生物聚合物衍生碳阴极设计高能量和稳定的 AZHBC 提供了一种方法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Applied Energy Materials
Materials Science-Materials Chemistry
CiteScore
10.30
自引率
6.20%
发文量
1368
期刊介绍:
ACS Applied Energy Materials is an interdisciplinary journal publishing original research covering all aspects of materials, engineering, chemistry, physics and biology relevant to energy conversion and storage. The journal is devoted to reports of new and original experimental and theoretical research of an applied nature that integrate knowledge in the areas of materials, engineering, physics, bioscience, and chemistry into important energy applications.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


