NiFe2O4 in MoSe2 Exhibits Bifunctional Water Oxidation and Oxygen Reduction (OER and ORR) Catalytic Reactions for Energy Applications
IF 5.4
3区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
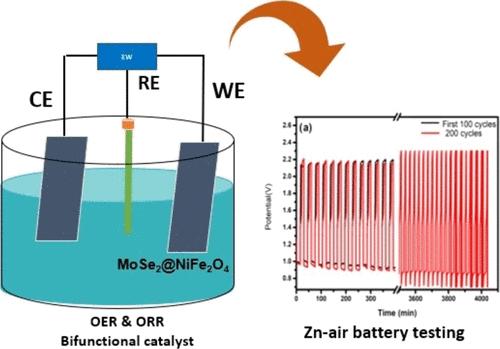
Highly active bifunctional oxygen evolution reaction (OER) and oxygen reduction reaction (ORR) catalysts made of nickel ferrite (NiFe2O4) supported on molybdenum diselenide (MoSe2) nanosheets have been rigorously studied in our present work. The OER activity evaluation was conducted in an alkaline solution for all catalysts. The MoSe2@NiFe2O4 (1:1) catalyst, which had shown superior activity compared to other catalysts, has an onset potential of 1.50 V vs reversible hydrogen electrode (RHE), similar to the state-of-the-art commercial IrO2. The ORR activity of the MoSe2@NiFe2O4 electrocatalyst exhibited an ORR onset potential of 0.83 V vs RHE. We report the MoSe2@NiFe2O4 bifunctional catalyst for noticeable activity in ORR and OER, with a potential difference (ΔE) of 0.92 V. In the accelerated test, after 5000 potential cycles, the MoSe2@NiFe2O4 (1:1) catalyst had about 86% retention of the ORR diffusion-limiting current density. The OER depicts a loss of around 70.6% after 2000 cycles, which is significantly lower than that of the state-of-the-art IrO2, deactivated after 2000 cycles. Harnessing the excellent bifunctionality of our catalyst, we tested the catalyst in the Zn–air battery, which depicts 300 cycles. The Zn–air battery long-term cycling test was performed at 20 mA cm–2 to assess the stability of the hybrid catalyst (30 min cycle–1), which exhibits a discharge voltage of 1.13 V and a charging voltage of 2.20 V. Considering the excellent bifunctional activity, the MoSe2@NiFe2O4 heterostructured composite is an exceptional candidate for energy storage applications.
MoSe2 中的 NiFe2O4 具有双功能水氧化和氧还原(OER 和 ORR)催化反应,可应用于能源领域
我们在本研究中严格研究了以二硒化钼 (MoSe2) 纳米片为载体的镍铁氧体 (NiFe2O4) 制成的高活性双功能氧进化反应 (OER) 和氧还原反应 (ORR) 催化剂。对所有催化剂的 OER 活性评估都是在碱性溶液中进行的。与其他催化剂相比,MoSe2@NiFe2O4(1:1)催化剂显示出更优越的活性,其对可逆氢电极(RHE)的起始电位为 1.50 V,与最先进的商用 IrO2 相似。MoSe2@NiFe2O4 电催化剂的 ORR 活性显示出对 RHE 的 ORR 起始电位为 0.83 V。在加速测试中,经过 5000 次电位循环后,MoSe2@NiFe2O4(1:1)催化剂的 ORR 扩散限制电流密度保持了约 86%。OER 在 2000 次循环后的损耗约为 70.6%,明显低于最先进的 IrO2(2000 次循环后失活)。利用我们催化剂的出色双功能性,我们在锌-空气电池中对催化剂进行了 300 次循环测试。为了评估混合催化剂的稳定性,我们在 20 mA cm-2 下进行了锌-空气电池长期循环测试(30 min cycle-1),结果显示放电电压为 1.13 V,充电电压为 2.20 V。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Applied Energy Materials
Materials Science-Materials Chemistry
CiteScore
10.30
自引率
6.20%
发文量
1368
期刊介绍:
ACS Applied Energy Materials is an interdisciplinary journal publishing original research covering all aspects of materials, engineering, chemistry, physics and biology relevant to energy conversion and storage. The journal is devoted to reports of new and original experimental and theoretical research of an applied nature that integrate knowledge in the areas of materials, engineering, physics, bioscience, and chemistry into important energy applications.
文献相关原料
| 公司名称 | 产品信息 | 采购帮参考价格 |
|---|
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


