Experimental Visualization of F-Ion Diffusion Pathways and Geometric Frustration-Induced Positional Disorder in CaF2–BaF2 Solid Electrolytes
IF 5.5
3区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Metastable CaF2–BaF2 compounds synthesized via high-energy ball milling or using thermal plasma are promising candidates for the fluoride-ion conducting solid electrolytes of all-solid-state fluoride batteries. The ionic conductivity of (CaF2)x(BaF2)1–x with x ≈ 0.5 is over 5 orders of magnitude greater than those of CaF2 and BaF2. However, the transport mechanism of fluoride ions in (CaF2)x(BaF2)1–x materials has not yet been fully elucidated. Herein, we have determined the conduction pathways of fluoride ions and local atomic configurations for (CaF2)0.48(BaF2)0.52 via a neutron diffraction analysis. Among various (CaF2)x(BaF2)1–x samples, (CaF2)0.48(BaF2)0.52 exhibits the highest conductivity and lower activation energy. The “–F1–□F–F1–” diffusion pathways of fluoride ions are identified via Rietveld refinement and a maximum entropy method, where F1 indicates a regular site for a fluoride ion (i.e., the (1/4, 1/4, 1/4) atomic position) within the T-unit composed of four Ca and/or Ba atoms, and the □F interstitial site is located at an off-center position in the O-unit composed of six Ca and/or Ba atoms. Moreover, reverse Monte Carlo modeling conducted using an atomic pair distribution function reveals the existence of geometric frustration-induced positional disorder with respect to Ca, Ba, and F atoms even at 150 K, which increases the mobility of fluoride ions in the “–F1–□F–F1–” diffusion pathways. The findings of this work may facilitate the development of solid electrolytes for next-generation all-solid-state rechargeable batteries with large-scale applications such as electric vehicles and residential energy storage systems.
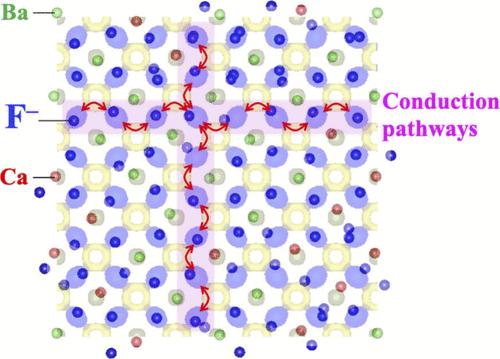
CaF2-BaF2 固体电解质中 F 离子扩散途径和几何挫折引起的位置紊乱的实验可视化
通过高能球磨或热等离子体合成的可蜕变 CaF2-BaF2 化合物有望成为全固态氟化物电池的氟离子导电固体电解质。(CaF2)x(BaF2)1-x 的离子电导率(x ≈ 0.5)比 CaF2 和 BaF2 高出 5 个数量级以上。然而,(CaF2)x(BaF2)1-x 材料中氟离子的传输机制尚未完全阐明。在此,我们通过中子衍射分析确定了(CaF2)0.48(BaF2)0.52的氟离子传导路径和局部原子构型。在各种(CaF2)x(BaF2)1-x 样品中,(CaF2)0.48(BaF2)0.52 的导电率最高,活化能较低。通过里特维尔德细化和最大熵法确定了氟离子的"-F1-□F-F1-"扩散路径,其中 F1 表示氟离子在由四个 Ca 原子和/或 Ba 原子组成的 T 单元中的常规位置(即(1/4,1/4,1/4)原子位置),而 □F 间隙位置则位于由六个 Ca 原子和/或 Ba 原子组成的 O 单元中的偏心位置。此外,利用原子对分布函数进行的反向蒙特卡罗建模显示,即使在 150 K 时,Ca、Ba 和 F 原子也存在几何挫折引起的位置紊乱,这增加了氟离子在"-F1-□F-F1-"扩散途径中的流动性。这项工作的发现可能会促进下一代全固态可充电电池固态电解质的开发,并大规模应用于电动汽车和住宅储能系统等领域。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Applied Energy Materials
Materials Science-Materials Chemistry
CiteScore
10.30
自引率
6.20%
发文量
1368
期刊介绍:
ACS Applied Energy Materials is an interdisciplinary journal publishing original research covering all aspects of materials, engineering, chemistry, physics and biology relevant to energy conversion and storage. The journal is devoted to reports of new and original experimental and theoretical research of an applied nature that integrate knowledge in the areas of materials, engineering, physics, bioscience, and chemistry into important energy applications.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


