Novel benzothiazole derivatives target the Gac/Rsm two-component system as antibacterial synergists against Pseudomonas aeruginosa infections
IF 14.7
1区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
The management of antibiotic-resistant, bacterial biofilm infections in skin wounds poses an increasingly challenging clinical scenario. Pseudomonas aeruginosa infection is difficult to eradicate because of biofilm formation and antibiotic resistance. In this study, we identified a new benzothiazole derivative compound, SN12 (IC50 = 43.3 nmol/L), demonstrating remarkable biofilm inhibition at nanomolar concentrations in vitro. In further activity assays and mechanistic studies, we formulated an unconventional strategy for combating P. aeruginosa-derived infections by targeting the two-component (Gac/Rsm) system. Furthermore, SN12 slowed the development of ciprofloxacin and tobramycin resistance. By using murine skin wound infection models, we observed that SN12 significantly augmented the antibacterial effects of three widely used antibiotics—tobramycin (100-fold), vancomycin (200-fold), and ciprofloxacin (1000-fold)—compared with single-dose antibiotic treatments for P. aeruginosa infection in vivo. The findings of this study suggest the potential of SN12 as a promising antibacterial synergist, highlighting the effectiveness of targeting the two-component system in treating challenging bacterial biofilm infections in humans.
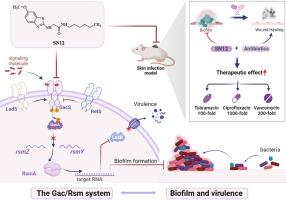
以 Gac/Rsm 双组分系统为靶点的新型苯并噻唑衍生物是铜绿假单胞菌感染的抗菌增效剂
由于生物膜的形成和抗生素的耐药性,很难根除皮肤伤口感染。在这项研究中,我们发现了一种新的苯并噻唑衍生物化合物(IC = 43.3 nmol/L),在纳摩尔浓度下具有显著的生物膜抑制作用。在进一步的活性测定和机理研究中,我们通过靶向双组分(Gac/Rsm)系统,制定了一种非常规的抗-源感染策略。此外,还减缓了环丙沙星和妥布霉素耐药性的发展。通过使用小鼠皮肤伤口感染模型,我们观察到,与单剂量抗生素治疗感染相比,三种广泛使用的抗生素--妥布霉素(100 倍)、万古霉素(200 倍)和环丙沙星(1000 倍)的抗菌效果显著增强。这项研究的结果表明,双组分系统有可能成为一种很有前景的抗菌增效剂,从而突出了以双组分系统为靶点治疗人类具有挑战性的细菌生物膜感染的有效性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Acta Pharmaceutica Sinica. B
Pharmacology, Toxicology and Pharmaceutics-General Pharmacology, Toxicology and Pharmaceutics
CiteScore
22.40
自引率
5.50%
发文量
1051
审稿时长
19 weeks
期刊介绍:
The Journal of the Institute of Materia Medica, Chinese Academy of Medical Sciences, and the Chinese Pharmaceutical Association oversees the peer review process for Acta Pharmaceutica Sinica. B (APSB).
Published monthly in English, APSB is dedicated to disseminating significant original research articles, rapid communications, and high-quality reviews that highlight recent advances across various pharmaceutical sciences domains. These encompass pharmacology, pharmaceutics, medicinal chemistry, natural products, pharmacognosy, pharmaceutical analysis, and pharmacokinetics.
A part of the Acta Pharmaceutica Sinica series, established in 1953 and indexed in prominent databases like Chemical Abstracts, Index Medicus, SciFinder Scholar, Biological Abstracts, International Pharmaceutical Abstracts, Cambridge Scientific Abstracts, and Current Bibliography on Science and Technology, APSB is sponsored by the Institute of Materia Medica, Chinese Academy of Medical Sciences, and the Chinese Pharmaceutical Association. Its production and hosting are facilitated by Elsevier B.V. This collaborative effort ensures APSB's commitment to delivering valuable contributions to the pharmaceutical sciences community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


