PKCα inhibitors promote breast cancer immune evasion by maintaining PD-L1 stability
IF 14.7
1区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
Protein kinase C α (PKCα) regulates diverse biological functions of cancer cells and is a promising therapeutic target. However, clinical trials of PKC-targeted therapies have not yielded satisfactory results. Recent studies have also indicated a tumor-suppressive role of PKCs via unclear molecular mechanisms. In this study, we found that PKCα inhibition enhances CD8+ T-cell-mediated tumor evasion and abolishes antitumor activity in immunocompetent mice. We further identified PKCα as a critical regulator of programmed cell death-ligand 1 (PD-L1) and found that it enhances T-cell-dependent antitumor immunity in breast cancer by interacting with PD-L1 and suppressing PD-L1 expression. We demonstrated that PKCα-mediated PD-L1 phosphorylation promotes PD-L1 degradation through β transducin repeat-containing protein. Notably, the efficacy of PKCα inhibitors was intensified by synergizing with anti-PD-L1 mAb therapy to boost antitumor T-cell immunity in vivo. Clinical analysis revealed that PKCα expression is positively correlated with T-cell function and the interferon-gamma signature in patients with breast cancer. This study demonstrated the antitumor capability of PKCα, identified potential therapeutic strategies to avoid tumor evasion via PKC-targeted therapies, and provided a proof of concept for targeting PKCα in combination with anti-PD-L1 mAb therapy as a potential therapeutic approach against breast cancer, especially TNBC.
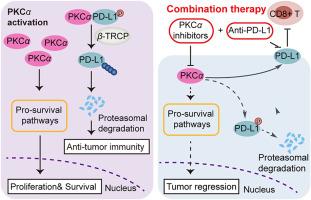
PKCα 抑制剂通过维持 PD-L1 的稳定性促进乳腺癌免疫逃避
蛋白激酶C(PKC)调节癌细胞的多种生物功能,是一个很有前景的治疗靶点。然而,PKC 靶向疗法的临床试验并未取得令人满意的结果。最近的研究还表明,PKCs 的抑制肿瘤作用的分子机制尚不清楚。在这项研究中,我们发现抑制PKC会增强CD8 T细胞介导的肿瘤逃避,并取消免疫功能正常小鼠的抗肿瘤活性。我们进一步确定了 PKC 是程序性细胞死亡配体 1(PD-L1)的关键调节因子,并发现它通过与 PD-L1 相互作用并抑制 PD-L1 的表达,增强了乳腺癌中 T 细胞依赖性抗肿瘤免疫。我们证实,PKC 介导的 PD-L1 磷酸化可通过含转导蛋白重复序列蛋白促进 PD-L1 降解。值得注意的是,PKC抑制剂与抗PD-L1 mAb疗法协同增强了抗肿瘤T细胞免疫的疗效。临床分析表明,PKC 的表达与乳腺癌患者的 T 细胞功能和干扰素-γ 特征呈正相关。这项研究证明了PKC的抗肿瘤能力,确定了避免肿瘤逃避PKC靶向疗法的潜在治疗策略,并提供了将PKC靶向疗法与抗PD-L1 mAb疗法相结合作为乳腺癌(尤其是TNBC)潜在治疗方法的概念验证。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Acta Pharmaceutica Sinica. B
Pharmacology, Toxicology and Pharmaceutics-General Pharmacology, Toxicology and Pharmaceutics
CiteScore
22.40
自引率
5.50%
发文量
1051
审稿时长
19 weeks
期刊介绍:
The Journal of the Institute of Materia Medica, Chinese Academy of Medical Sciences, and the Chinese Pharmaceutical Association oversees the peer review process for Acta Pharmaceutica Sinica. B (APSB).
Published monthly in English, APSB is dedicated to disseminating significant original research articles, rapid communications, and high-quality reviews that highlight recent advances across various pharmaceutical sciences domains. These encompass pharmacology, pharmaceutics, medicinal chemistry, natural products, pharmacognosy, pharmaceutical analysis, and pharmacokinetics.
A part of the Acta Pharmaceutica Sinica series, established in 1953 and indexed in prominent databases like Chemical Abstracts, Index Medicus, SciFinder Scholar, Biological Abstracts, International Pharmaceutical Abstracts, Cambridge Scientific Abstracts, and Current Bibliography on Science and Technology, APSB is sponsored by the Institute of Materia Medica, Chinese Academy of Medical Sciences, and the Chinese Pharmaceutical Association. Its production and hosting are facilitated by Elsevier B.V. This collaborative effort ensures APSB's commitment to delivering valuable contributions to the pharmaceutical sciences community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


