First-in-human study of DP303c, a HER2-targeted antibody-drug conjugate in patients with HER2 positive solid tumors
IF 6.8
1区 医学
Q1 ONCOLOGY
引用次数: 0
Abstract
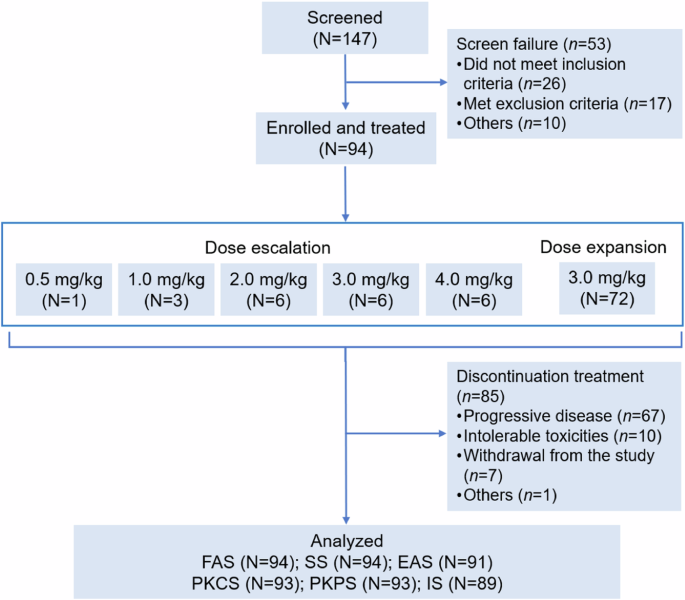
DP303c is a HER2-targeted ADC with a cleavable linker-MMAE payload. Previous in vitro studies demonstrated that DP303c showed similar or better antitumor activity than T-DM1 in xenograft models. This was a multicenter, dose escalation and dose expansion phase 1 study in China. Eligible patients were 18-75 years old with HER2-positive advanced solid tumors who were unable to benefit from standard therapy. DP303c was administered intravenously every 3 weeks, with accelerated titration at lower dose of 0.5 mg/kg and 3 + 3 design with dose levels of 1.0, 2.0, 3.0 or 4.0 mg/kg at dose escalation part, followed by the selected dose level at dose expansion part. The primary endpoints were safety and tolerability, as well as identification of recommended phase 2 dose. As of Feb 28, 2023, 94 patients were enrolled and received DP303c (dose escalation: n = 22; dose expansion: n = 72), of whom 68 patients had breast cancer. One dose limiting toxicity (Grade 3 eye pain) was observed at 4.0 mg/kg dose, and the maximum tolerated dose was not reached. The most common treatment-related adverse events at grade 3 or higher were blurred vison (16.0%), dry eye (6.4%), and peripheral neuropathy (5.3%). No treatment-related death occurred. Overall, among 91 efficacy evaluable patients, 39 patients (42.9%) achieved an objective response. Disease control was observed in 62 patients (68.1%). In 66 efficacy evaluable patients with breast cancer, 34 patients achieved an objective response (51.5%). Disease control was achieved in 51 patients (77.3%). Median PFS was 6.4 months. On a molar basis, DP303c Cmax at 3.0 mg/kg doses was 132-folder higher than that for free MMAE. DP303c demonstrated promising anti-tumor activity with acceptable safety in patients with pre-treated advanced HER2 positive solid tumors, especially in breast cancer. Based on safety and efficacy results, 3.0 mg/kg Q3W was determined as recommended phase 2 dose for DP303c. (Trial registration: ClinicalTrials.gov Identifier: NCT04146610).
针对HER2阳性实体瘤患者的HER2靶向抗体-药物共轭物DP303c首次人体研究
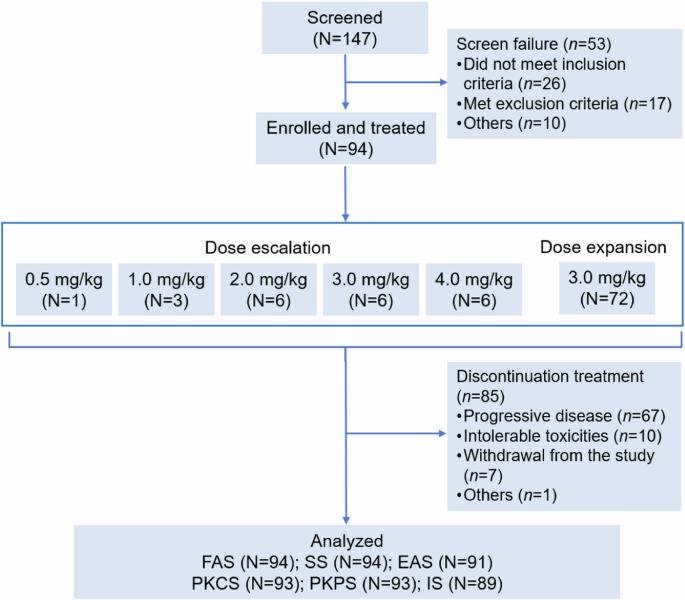
DP303c 是一种 HER2 靶向 ADC,具有可裂解连接体-MMAE 有效载荷。之前的体外研究表明,DP303c在异种移植模型中显示出与T-DM1相似或更好的抗肿瘤活性。这是一项在中国进行的多中心、剂量递增和剂量扩大的一期研究。符合条件的患者年龄为18-75岁,患有HER2阳性晚期实体瘤,且无法从标准疗法中获益。DP303c每3周静脉给药1次,以0.5 mg/kg的低剂量加速滴定,剂量升级部分采用3+3设计,剂量水平为1.0、2.0、3.0或4.0 mg/kg,随后在剂量扩展部分采用选定的剂量水平。主要终点是安全性和耐受性,以及确定第二阶段的推荐剂量。截至2023年2月28日,共有94名患者入组并接受了DP303c治疗(剂量升级:22人;剂量扩大:72人),其中68名患者患有乳腺癌。4.0毫克/千克剂量时出现了一种剂量限制性毒性(3级眼痛),未达到最大耐受剂量。最常见的 3 级或以上治疗相关不良反应是视力模糊(16.0%)、干眼症(6.4%)和周围神经病变(5.3%)。没有发生与治疗相关的死亡事件。总体而言,在 91 名可进行疗效评估的患者中,有 39 名患者(42.9%)获得了客观反应。62名患者(68.1%)病情得到控制。在 66 名可进行疗效评估的乳腺癌患者中,34 名患者(51.5%)获得了客观应答。51名患者(77.3%)病情得到控制。中位生存期为 6.4 个月。按摩尔计算,DP303c 在 3.0 mg/kg 剂量时的 Cmax 比游离 MMAE 高 132 倍。DP303c 在晚期 HER2 阳性实体瘤(尤其是乳腺癌)预处理患者中表现出良好的抗肿瘤活性和可接受的安全性。根据安全性和有效性结果,DP303c的第二阶段推荐剂量为3.0 mg/kg Q3W。(试验注册:ClinicalTrials.gov Identifier:NCT04146610)。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

NPJ Precision Oncology
ONCOLOGY-
CiteScore
9.90
自引率
1.30%
发文量
87
审稿时长
18 weeks
期刊介绍:
Online-only and open access, npj Precision Oncology is an international, peer-reviewed journal dedicated to showcasing cutting-edge scientific research in all facets of precision oncology, spanning from fundamental science to translational applications and clinical medicine.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


