GAD1 ameliorates glioma progression through regulating cuproptosis via RAS/MAPK pathway
Abstract
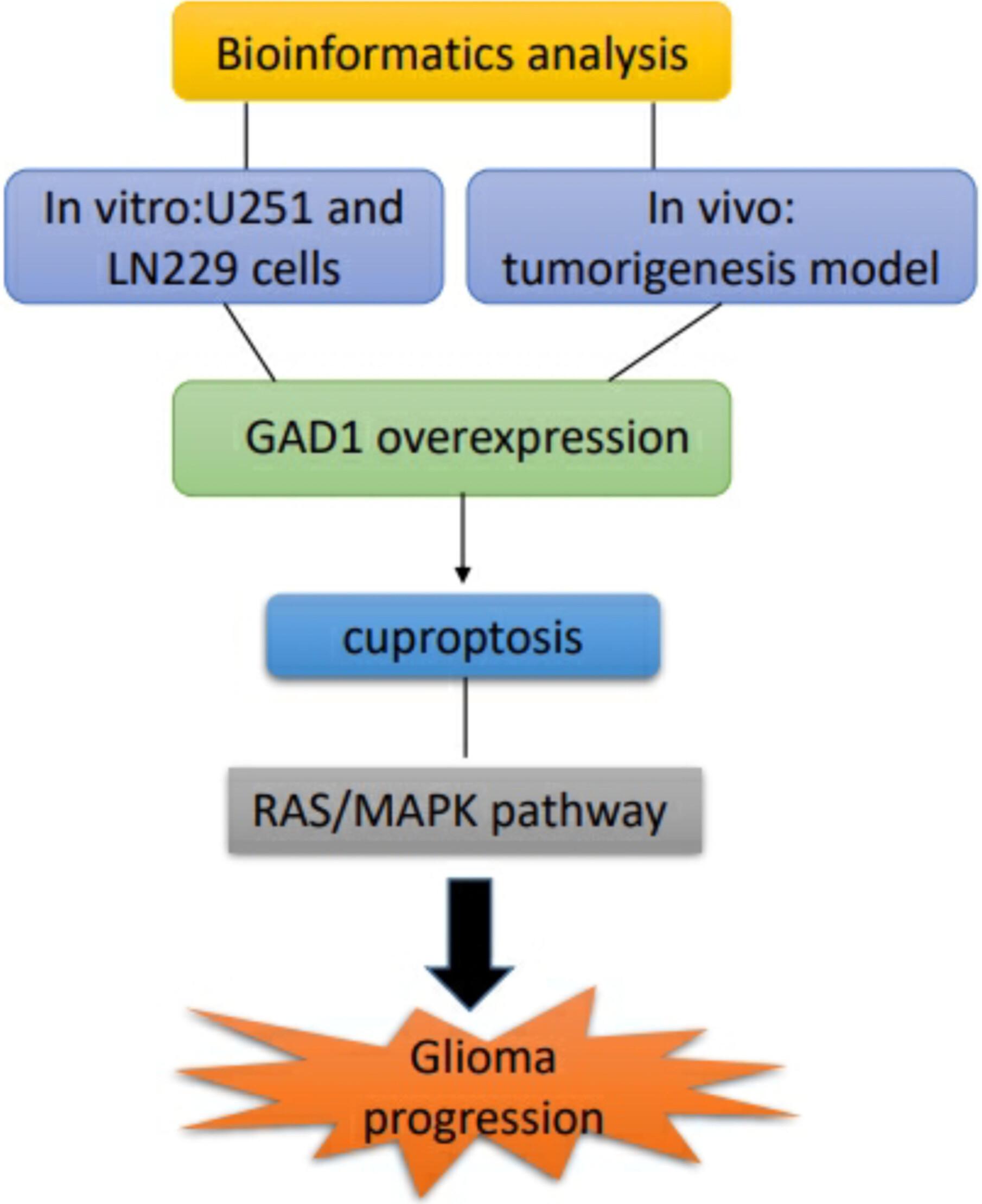
Glioma represents a primary malignant tumor occurring in the central nervous system. Glutamate decarboxylase (GAD1) plays a significant role in tumor development; however, its function of GAD1 and underlying mechanisms in glioma progression remain unclear. Differentially expressed genes (DEGs) obtained from the GSE12657 and GSE15209 datasets that intersected with cuproptosis-related genes and pivot genes were identified using comprehensive bioinformatics methods. The elesclomol (ES) treatment was used to induce cuproptosis in U251 cells, which was validated by detecting intracellular copper levels and cuproptosis marker expression. Lentivirus-mediated gene overexpression was performed to explore the effects of GAD1 using functional assays in vitro and in a mouse xenograft model. The RAS agonist ML098 was used to verify the effect of GAD1 on the RAS/MAPK pathway in glioma cells. A total of 87 cuproptosis-related DEGs and seven hub genes were obtained, with five genes upregulated and two were downregulated in gliomas. Overexpression of GAD1 inhibited proliferation, invasion, and migration, promoted apoptosis of glioma cells, and suppressed tumorigenesis in vivo. In addition, GAD1 overexpression enhanced the sensitivity of glioma cells to cuproptosis. Additionally, ML098 treatment attenuated the inhibitory effect of GAD1 overexpression on the malignant phenotype of ES-treated cells. GAD1 plays an anti-oncogenic role in glioma by regulating apoptosis via inhibition of the RAS/MAPK pathway.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


