A Solid-State Electrolyte Facilitates Acidic CO2 Electrolysis without Alkali Metal Cations by Regulating Proton Transport
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
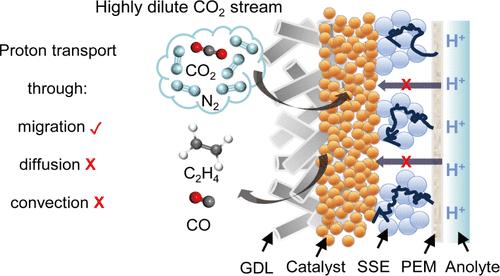
Electrochemical CO2 reduction (CO2R) in acidic media provides a pathway to curtail CO2 losses by suppressing the formation of (bi)carbonates. In such systems, a high concentration of alkali metal cations is necessary for mitigating the proton-rich environment and suppressing the competing hydrogen evolution reaction. However, a high cation concentration also promotes salt precipitation within the gas diffusion layer, resulting in poor system durability. Here, we resolve this conundrum by replacing the liquid catholyte with a solid-state proton conductor to regulate H+ transport. This is postulated to allow for a locally alkaline environment at the cathode, enabling selective CO2R even without alkali metal cations. We show that this strategy is effective over a broad range of catalyst systems. For instance, we achieve an 87% CO faradaic efficiency (FE) at 300 mA/cm2 using a composite nanoporous Au and single-atom Ni catalyst, with 0.25 M H2SO4 as the anolyte. Stable operation over 110 h and a high single-pass carbon efficiency of 82.8% were also successfully demonstrated. Importantly, we find that this solid-state system is also particularly effective at converting dilute feedstock (5% CO2) with a CO FE of 47.7%, a factor of 16.4 times higher than a conventional system. Our results introduce a simple yet effective design approach for developing efficient acidic CO2R electrolyzers.
固态电解质通过调节质子传输促进无碱金属阳离子的酸性二氧化碳电解
酸性介质中的电化学二氧化碳还原(CO2R)提供了一条通过抑制(双)碳酸盐的形成来减少二氧化碳损失的途径。在此类系统中,高浓度的碱金属阳离子是缓解富质子环境和抑制竞争性氢进化反应的必要条件。然而,高浓度的阳离子也会促进气体扩散层内的盐沉淀,导致系统耐久性差。在这里,我们用固态质子导体取代液态阴解质来调节 H+ 的传输,从而解决了这一难题。据推测,这样可以在阴极形成局部碱性环境,即使没有碱金属阳离子也能实现选择性 CO2R。我们的研究表明,这种策略在多种催化剂体系中都很有效。例如,我们使用了一种复合纳米多孔金和单原子镍催化剂,以 0.25 M H2SO4 为电解质,在 300 mA/cm2 的条件下实现了 87% 的 CO 法拉第效率 (FE)。此外,该催化剂还能稳定运行 110 小时,单程碳效率高达 82.8%。重要的是,我们发现这种固态系统对稀释原料(5% CO2)的转化也特别有效,CO FE 为 47.7%,是传统系统的 16.4 倍。我们的研究成果为开发高效酸性 CO2R 电解槽提供了一种简单而有效的设计方法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


