Synthesis of α-Aminonitriles via Ammonium-Catalyzed Reactions of Aminoacetonitrile
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
α-Aminonitriles are not only broadly useful building blocks but also structural motifs in bioactive molecules. The Strecker reaction is one of the most widely used methods for α-aminonitrile synthesis. However, a severe drawback in Strecker reactions is the required use of a stoichiometric amount of toxic cyanation reagents. Thus, the development of a greener and widely applicable method for the synthesis of aminonitriles from readily available starting materials presents an important yet unmet challenge. We developed a general and new method for the synthesis of aminonitriles from readily available aminoacetonitrile. This method utilized off-the-shelf ammonium salts as catalysts, tolerated air and moisture, and avoided the use of cyanation reagents, which rendered it a greener alternative to the widely practiced Strecker reaction approach. We further illustrated that chiral ammonium-catalyzed asymmetric reactions of N-arylidene aminoacetonitriles could provide chiral α-tertiary and α-quaternary aminonitriles and α-aminonitriles bearing two continuous stereocenters.
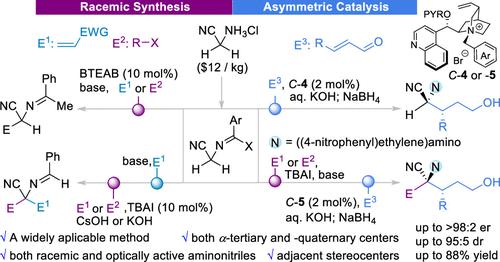
通过氨基乙腈的氨催化反应合成 α-氨基腈化合物
α-氨基腈不仅是用途广泛的构筑砌块,也是生物活性分子的结构基团。Strecker 反应是最广泛使用的 α-氨基腈合成方法之一。然而,Strecker 反应的一个严重缺点是需要使用一定量的有毒氰化试剂。因此,开发一种更环保、适用范围更广的方法,利用容易获得的起始材料合成氨腈,是一项重要但尚未解决的挑战。我们开发了一种利用现成的氨基乙腈合成氨腈的通用新方法。该方法利用现成的铵盐作为催化剂,耐空气和湿气,避免使用氰化试剂,是广泛使用的 Strecker 反应方法的绿色替代方法。我们进一步说明,手性铵催化的 N-芳基氨基乙腈不对称反应可以提供手性 α-叔氨基乙腈和 α-季氨基乙腈,以及带有两个连续立体中心的 α-氨基乙腈。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


