Signal flow in the NMDA receptor–dependent phosphoproteome regulates postsynaptic plasticity for aversive learning
IF 6.6
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
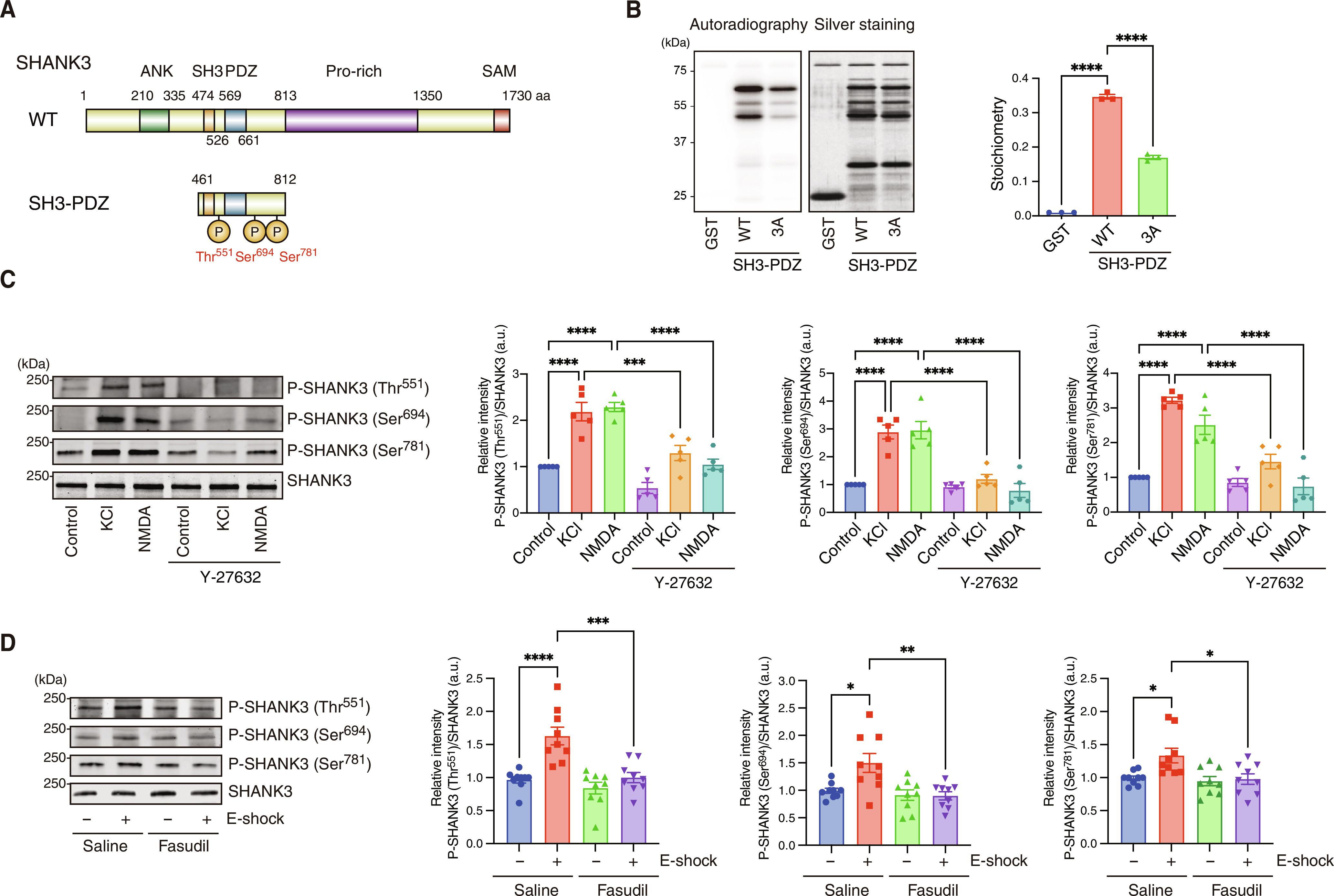
Structural plasticity of dendritic spines in the nucleus accumbens (NAc) is crucial for learning from aversive experiences. Activation of NMDA receptors (NMDARs) stimulates Ca2+-dependent signaling that leads to changes in the actin cytoskeleton, mediated by the Rho family of GTPases, resulting in postsynaptic remodeling essential for learning. We investigated how phosphorylation events downstream of NMDAR activation drive the changes in synaptic morphology that underlie aversive learning. Large-scale phosphoproteomic analyses of protein kinase targets in mouse striatal/accumbal slices revealed that NMDAR activation resulted in the phosphorylation of 194 proteins, including RhoA regulators such as ARHGEF2 and ARHGAP21. Phosphorylation of ARHGEF2 by the Ca2+-dependent protein kinase CaMKII enhanced its RhoGEF activity, thereby activating RhoA and its downstream effector Rho-associated kinase (ROCK/Rho-kinase). Further phosphoproteomic analysis identified 221 ROCK targets, including the postsynaptic scaffolding protein SHANK3, which is crucial for its interaction with NMDARs and other postsynaptic scaffolding proteins. ROCK-mediated phosphorylation of SHANK3 in the NAc was essential for spine growth and aversive learning. These findings demonstrate that NMDAR activation initiates a phosphorylation cascade crucial for learning and memory.
依赖于 NMDA 受体的磷酸蛋白体的信号流调节突触后的可塑性,从而促进厌恶性学习
脑内凹凸核(NAc)树突棘的结构可塑性对于从厌恶经验中学习至关重要。NMDA 受体(NMDAR)的激活会刺激 Ca2+ 依赖性信号传导,从而导致肌动蛋白细胞骨架在 Rho 系列 GTP 酶的介导下发生变化,导致对学习至关重要的突触后重塑。我们研究了 NMDAR 激活下游的磷酸化事件如何驱动突触形态的变化,而突触形态的变化是厌恶性学习的基础。对小鼠纹状体/丘脑切片中的蛋白激酶靶点进行的大规模磷酸化蛋白质组学分析表明,NMDAR 的激活导致了 194 种蛋白质的磷酸化,其中包括 RhoA 调控因子,如 ARHGEF2 和 ARHGAP21。钙离子依赖性蛋白激酶 CaMKII 对 ARHGEF2 的磷酸化增强了其 RhoGEF 活性,从而激活了 RhoA 及其下游效应物 Rho-associated 激酶(ROCK/Rho-激酶)。进一步的磷酸化蛋白组学分析确定了 221 个 ROCK 靶点,包括突触后支架蛋白 SHANK3,该蛋白对 ROCK 与 NMDAR 及其他突触后支架蛋白的相互作用至关重要。ROCK 介导的 SHANK3 在 NAc 中的磷酸化对脊柱生长和厌恶学习至关重要。这些研究结果表明,NMDAR的激活启动了对学习和记忆至关重要的磷酸化级联。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Science Signaling
BIOCHEMISTRY & MOLECULAR BIOLOGY-CELL BIOLOGY
CiteScore
9.50
自引率
0.00%
发文量
148
审稿时长
3-8 weeks
期刊介绍:
"Science Signaling" is a reputable, peer-reviewed journal dedicated to the exploration of cell communication mechanisms, offering a comprehensive view of the intricate processes that govern cellular regulation. This journal, published weekly online by the American Association for the Advancement of Science (AAAS), is a go-to resource for the latest research in cell signaling and its various facets.
The journal's scope encompasses a broad range of topics, including the study of signaling networks, synthetic biology, systems biology, and the application of these findings in drug discovery. It also delves into the computational and modeling aspects of regulatory pathways, providing insights into how cells communicate and respond to their environment.
In addition to publishing full-length articles that report on groundbreaking research, "Science Signaling" also features reviews that synthesize current knowledge in the field, focus articles that highlight specific areas of interest, and editor-written highlights that draw attention to particularly significant studies. This mix of content ensures that the journal serves as a valuable resource for both researchers and professionals looking to stay abreast of the latest advancements in cell communication science.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


