Spatial single-cell isotope tracing reveals heterogeneity of de novo fatty acid synthesis in cancer
IF 18.9
1区 医学
Q1 ENDOCRINOLOGY & METABOLISM
引用次数: 0
Abstract
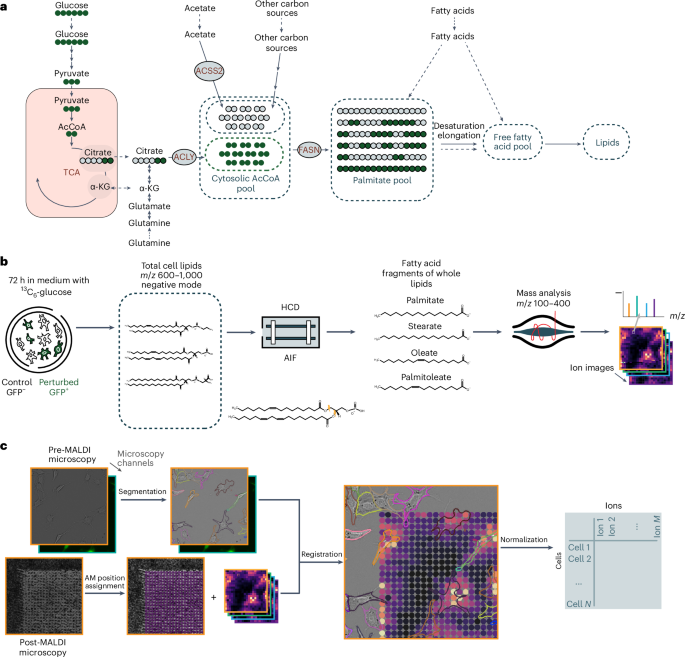
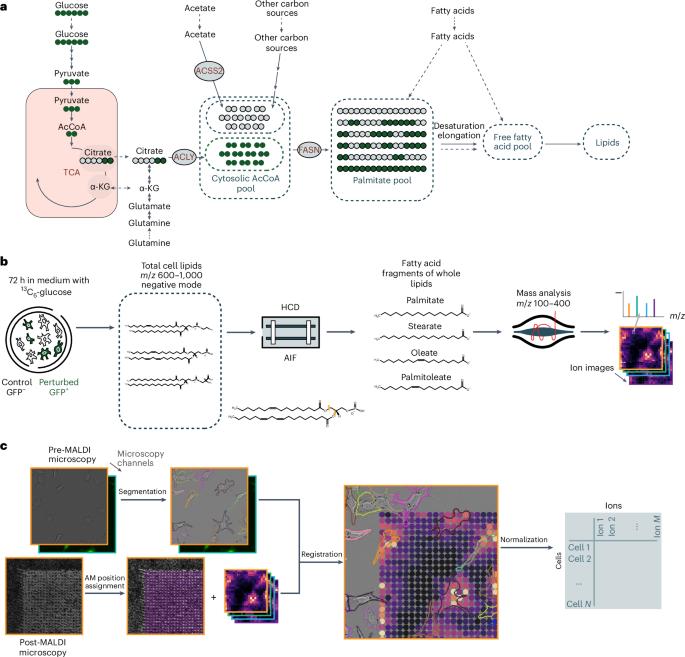
While heterogeneity is a key feature of cancer, understanding metabolic heterogeneity at the single-cell level remains a challenge. Here we present 13C-SpaceM, a method for spatial single-cell isotope tracing that extends the previously published SpaceM method with detection of 13C6-glucose-derived carbons in esterified fatty acids. We validated 13C-SpaceM on spatially heterogeneous models using liver cancer cells subjected to either normoxia-hypoxia or ATP citrate lyase depletion. This revealed substantial single-cell heterogeneity in labelling of the lipogenic acetyl-CoA pool and in relative fatty acid uptake versus synthesis hidden in bulk analyses. Analysing tumour-bearing brain tissue from mice fed a 13C6-glucose-containing diet, we found higher glucose-dependent synthesis of saturated fatty acids and increased elongation of essential fatty acids in tumours compared with healthy brains. Furthermore, our analysis uncovered spatial heterogeneity in lipogenic acetyl-CoA pool labelling in tumours. Our method enhances spatial probing of metabolic activities in single cells and tissues, providing insights into fatty acid metabolism in homoeostasis and disease. Buglakova et al. present 13C-SpaceM, a method that combines stable isotope tracing with imaging mass spectrometry thus enabling spatial analysis of lipid dynamics with near-single-cell resolution in tissues.
空间单细胞同位素追踪揭示癌症中脂肪酸合成的异质性
虽然异质性是癌症的一个主要特征,但在单细胞水平上理解代谢异质性仍然是一项挑战。我们在此介绍 13C-SpaceM,这是一种用于空间单细胞同位素追踪的方法,它扩展了之前发表的 SpaceM 方法,检测酯化脂肪酸中 13C6 葡萄糖衍生的碳。我们使用常氧-缺氧或 ATP 枸橼酸裂解酶耗竭的肝癌细胞,在空间异质性模型上验证了 13C-SpaceM 方法。结果表明,在大量分析中隐藏的脂肪生成乙酰-CoA 池的标记和相对脂肪酸摄取与合成中存在大量单细胞异质性。通过分析喂食含 13C6 葡萄糖饮食的小鼠肿瘤脑组织,我们发现与健康大脑相比,肿瘤中饱和脂肪酸的葡萄糖依赖性合成更高,必需脂肪酸的伸长率也更高。此外,我们的分析还发现了肿瘤中生脂乙酰-CoA池标记的空间异质性。我们的方法增强了对单细胞和组织中代谢活动的空间探测,为了解脂肪酸在体内平衡和疾病中的代谢情况提供了线索。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature metabolism
ENDOCRINOLOGY & METABOLISM-
CiteScore
27.50
自引率
2.40%
发文量
170
期刊介绍:
Nature Metabolism is a peer-reviewed scientific journal that covers a broad range of topics in metabolism research. It aims to advance the understanding of metabolic and homeostatic processes at a cellular and physiological level. The journal publishes research from various fields, including fundamental cell biology, basic biomedical and translational research, and integrative physiology. It focuses on how cellular metabolism affects cellular function, the physiology and homeostasis of organs and tissues, and the regulation of organismal energy homeostasis. It also investigates the molecular pathophysiology of metabolic diseases such as diabetes and obesity, as well as their treatment. Nature Metabolism follows the standards of other Nature-branded journals, with a dedicated team of professional editors, rigorous peer-review process, high standards of copy-editing and production, swift publication, and editorial independence. The journal has a high impact factor, has a certain influence in the international area, and is deeply concerned and cited by the majority of scholars.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


