Mitochondria-related HSDL2 is a potential biomarker in temporal lobe epilepsy by modulating astrocytic lipid metabolism
IF 5.6
2区 医学
Q1 CLINICAL NEUROLOGY
引用次数: 0
Abstract
Temporal lobe epilepsy (TLE) is the most prevalent type of focal epilepsy in adults. While comprehensive bioinformatics analyses have facilitated the identification of novel biomarkers in animal models, similar efforts are limited for TLE patients. In the current study, a comprehensive analysis using human transcriptomics datasets GSE205661, GSE190451, and GSE186334 was conducted to reveal differentially expressed genes related to mitochondria (Mito-DEGs). Protein-protein interaction (PPI) network and Least Absolute Shrinkage and Selection Operator (LASSO) regression analyses were performed to identify hub genes. Additional GSE127871 and GSE255223 were utilized to establish the association with hippocampal sclerosis (HS) and seizure frequency, respectively. Single-cell RNA analysis, functional investigation, and clinical verification were conducted. Herein, we reported that the Mito-DEGs in human TLE were significantly enriched in metabolic processes. Through PPI and LASSO analysis, HSDL2 was identified as the hub gene, of which diagnostic potential was further confirmed using independent datasets, animal models, and clinical validation. Subsequent single-cell and functional analyses revealed that HSDL2 expression was enriched and upregulated in response to excessive lipid accumulation in astrocytes. Additionally, the diagnostic efficiency of blood HSDL2 was verified in Qilu cohort. Together, our findings highlight the translational potential of HSDL2 as a biomarker and provide a novel therapeutic perspective for human TLE.
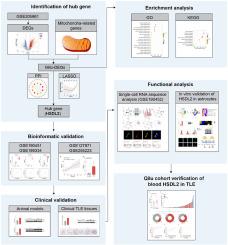
线粒体相关 HSDL2 通过调节星形胶质细胞的脂质代谢,成为颞叶癫痫的潜在生物标志物。
颞叶癫痫(TLE)是成人中最常见的局灶性癫痫类型。虽然全面的生物信息学分析有助于鉴定动物模型中的新型生物标志物,但对于颞叶癫痫患者来说,类似的工作还很有限。本研究利用人类转录组学数据集 GSE205661、GSE190451 和 GSE186334 进行了综合分析,以揭示与线粒体相关的差异表达基因(Mito-DEGs)。进行了蛋白质-蛋白质相互作用(PPI)网络和最小绝对收缩和选择操作器(LASSO)回归分析,以确定枢纽基因。另外还利用 GSE127871 和 GSE255223 分别确定了与海马硬化症(HS)和癫痫发作频率的关联。我们还进行了单细胞 RNA 分析、功能调查和临床验证。在此,我们报告了人类 TLE 中的 Mito-DEGs 在代谢过程中显著富集。通过PPI和LASSO分析,HSDL2被确定为枢纽基因,其诊断潜力通过独立数据集、动物模型和临床验证得到了进一步证实。随后的单细胞和功能分析显示,HSDL2的表达在星形胶质细胞脂质过度积累时丰富和上调。此外,血液 HSDL2 的诊断效率也在齐鲁队列中得到了验证。总之,我们的研究结果凸显了HSDL2作为生物标志物的转化潜力,并为人类TLE提供了新的治疗视角。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Neurotherapeutics
医学-神经科学
CiteScore
11.00
自引率
3.50%
发文量
154
审稿时长
6-12 weeks
期刊介绍:
Neurotherapeutics® is the journal of the American Society for Experimental Neurotherapeutics (ASENT). Each issue provides critical reviews of an important topic relating to the treatment of neurological disorders written by international authorities.
The Journal also publishes original research articles in translational neuroscience including descriptions of cutting edge therapies that cross disciplinary lines and represent important contributions to neurotherapeutics for medical practitioners and other researchers in the field.
Neurotherapeutics ® delivers a multidisciplinary perspective on the frontiers of translational neuroscience, provides perspectives on current research and practice, and covers social and ethical as well as scientific issues.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


