Efferocytosis drives a tryptophan metabolism pathway in macrophages to promote tissue resolution
IF 18.9
1区 医学
Q1 ENDOCRINOLOGY & METABOLISM
引用次数: 0
Abstract
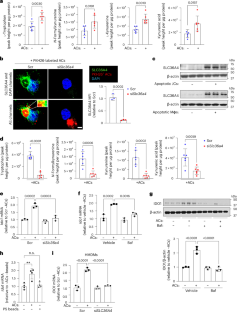
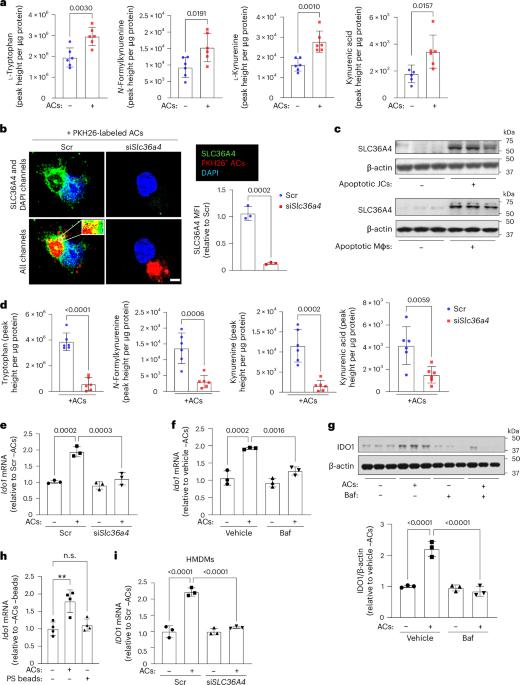
Macrophage efferocytosis prevents apoptotic cell (AC) accumulation and triggers inflammation-resolution pathways. The mechanisms linking efferocytosis to resolution often involve changes in macrophage metabolism, but many gaps remain in our understanding of these processes. We now report that efferocytosis triggers an indoleamine 2,3-dioxygenase-1 (IDO1)-dependent tryptophan (Trp) metabolism pathway that promotes several key resolution processes, including the induction of pro-resolving proteins, such interleukin-10, and further enhancement of efferocytosis. The process begins with upregulation of Trp transport and metabolism, and it involves subsequent activation of the aryl hydrocarbon receptor (AhR) by the Trp metabolite kynurenine (Kyn). Through these mechanisms, macrophage IDO1 and AhR contribute to a proper resolution response in several different mouse models of efferocytosis-dependent tissue repair, notably during atherosclerosis regression induced by plasma low-density lipoprotein (LDL) lowering. These findings reveal an integrated metabolism programme in macrophages that links efferocytosis to resolution, with possible therapeutic implications for non-resolving chronic inflammatory diseases, notably atherosclerosis. Sukka et al. delineate a metabolic pathway in macrophages that involves tryptophan uptake and metabolism to drive efferocytosis and subsequent resolution in the context of inflammation.
吞噬作用驱动巨噬细胞中的色氨酸代谢途径,促进组织修复
巨噬细胞的排泄可防止凋亡细胞(AC)的积累,并触发炎症缓解途径。将渗出与炎症缓解联系起来的机制往往涉及巨噬细胞新陈代谢的变化,但我们对这些过程的了解仍存在许多空白。我们现在报告说,渗出会触发一个依赖于色氨酸(Trp)代谢途径的吲哚胺 2,3-二氧合酶-1(IDO1),该途径会促进几个关键的消解过程,包括诱导白细胞介素-10 等促进消解的蛋白,以及进一步增强渗出。这一过程始于 Trp 转运和代谢的上调,随后涉及 Trp 代谢产物犬尿氨酸(Kyn)对芳基烃受体(AhR)的激活。通过这些机制,巨噬细胞 IDO1 和 AhR 在几种不同的依赖于组织修复的流出小鼠模型中,特别是在降低血浆低密度脂蛋白(LDL)诱导的动脉粥样硬化消退过程中,有助于适当的解决反应。这些发现揭示了巨噬细胞中的综合新陈代谢程序,该程序将渗出与溶解联系在一起,可能对非溶解性慢性炎症性疾病,特别是动脉粥样硬化具有治疗意义。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature metabolism
ENDOCRINOLOGY & METABOLISM-
CiteScore
27.50
自引率
2.40%
发文量
170
期刊介绍:
Nature Metabolism is a peer-reviewed scientific journal that covers a broad range of topics in metabolism research. It aims to advance the understanding of metabolic and homeostatic processes at a cellular and physiological level. The journal publishes research from various fields, including fundamental cell biology, basic biomedical and translational research, and integrative physiology. It focuses on how cellular metabolism affects cellular function, the physiology and homeostasis of organs and tissues, and the regulation of organismal energy homeostasis. It also investigates the molecular pathophysiology of metabolic diseases such as diabetes and obesity, as well as their treatment. Nature Metabolism follows the standards of other Nature-branded journals, with a dedicated team of professional editors, rigorous peer-review process, high standards of copy-editing and production, swift publication, and editorial independence. The journal has a high impact factor, has a certain influence in the international area, and is deeply concerned and cited by the majority of scholars.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


