Long term safety and outcomes after atrial shunting for heart failure with preserved or mildly reduced ejection fraction: 5-year and 3-year follow-up in the REDUCE LAP-HF I and II trials
IF 3.7
2区 医学
Q1 CARDIAC & CARDIOVASCULAR SYSTEMS
引用次数: 0
Abstract
Background
There is a little evidence regarding long-term safety and efficacy for atrial shunt devices in heart failure (HF).
Methods
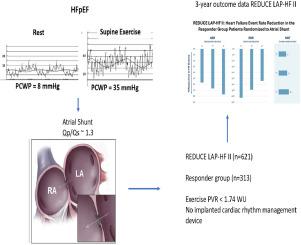
The REDUCE LAP-HF I (n = 44) and II (n = 621) trials (RCT-I and -II) were multicenter, randomized, sham-controlled trials of patients with HF and ejection fraction >40%. Outcome data were analyzed from RCT-I, a mechanistic trial with 5-year follow-up, and RCT-II, a pivotal trial identifying a responder group (n = 313) defined by exercise PVR <1.74 WU and no cardiac rhythm management device with 3-year follow-up.
Results
At 5 years in RCT I, there were no differences in cardiovascular (CV) mortality, HF events, embolic stroke, or new-onset atrial fibrillation between groups. After 3 years in RCT II, there was no difference in the primary outcome (hierarchical composite of CV mortality, stroke, HF events, and KCCQ) between shunt and sham in the overall trial. Compared to sham, those with responder characteristics in RCT-II had a better outcome with shunt (win ratio 1.6 [95% CI 1.2-2.2], P = .006; 44% reduction in HF events [shunt 9 vs. control 16 per 100 patient-years], P = .005; and greater improvement in KCCQ overall summary score [+17.9 ± 20.0 vs. +7.6 ± 20.4], P < .001), while nonresponders had significantly more HF events. Shunt treatment at 3 years was associated with a higher rate of ischemic stroke (3.2% vs. 0%, 95% CI 2%-6.1%, P = .032) and lower incidence of worsening kidney dysfunction (10.7% vs. 19.3%, P = .041).
Conclusions
With up to 5 years of follow up, adverse events were low in patients receiving atrial shunts. In the responder group, atrial shunt treatment was associated with a significantly lower HF event rate and improved KCCQ compared to sham through 3 years of follow-up.
Clinicaltrials.gov registration:
NCT02600234, NCT03088033.
射血分数保留或轻度降低的心力衰竭患者进行心房分流术后的长期安全性和疗效:REDUCE LAP-HF I 和 II 试验的 5 年和 3 年随访。
背景:关于心房分流装置在心力衰竭(HF)患者中的长期安全性和有效性,目前证据不足:关于心房分流装置在心力衰竭(HF)中的长期安全性和疗效,目前证据不足:REDUCE LAP-HF I (n=44) 和 II (n=621) 试验(RCT-I 和 -II)是针对射血分数大于 40% 的心力衰竭患者进行的多中心、随机、假对照试验。对 RCT-I 和 RCT-II 的结果数据进行了分析,RCT-I 是一项随访 5 年的机理试验,而 RCT-II 则是一项关键性试验,根据运动 PVR 确定了反应组(313 人):在 RCT I 的 5 年随访中,各组之间在心血管(CV)死亡率、高血压事件、栓塞性中风或新发心房颤动方面没有差异。RCT II 试验 3 年后,在整个试验中,分流组与假体组的主要结果(CV 死亡率、中风、高频事件和 KCCQ 的分层复合结果)没有差异。与假体相比,RCT-II 中具有响应者特征的患者使用分流术的疗效更好(获胜比为 1.6 [95% CI 1.2-2.2],P=0.006;高频事件减少 44%[每 100 患者年分流 9 例与对照组 16 例],P=0.005;KCCQ 总分改善更大[+17.9±20.0 与 +7.6±20.4],PC 结论:在长达5年的随访中,接受心房分流术的患者不良事件较少。在随访 3 年的应答组中,心房分流治疗与假治疗相比,可显著降低 HF 事件发生率并改善 KCCQ:NCT02600234、NCT03088033。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

American heart journal
医学-心血管系统
CiteScore
8.20
自引率
2.10%
发文量
214
审稿时长
38 days
期刊介绍:
The American Heart Journal will consider for publication suitable articles on topics pertaining to the broad discipline of cardiovascular disease. Our goal is to provide the reader primary investigation, scholarly review, and opinion concerning the practice of cardiovascular medicine. We especially encourage submission of 3 types of reports that are not frequently seen in cardiovascular journals: negative clinical studies, reports on study designs, and studies involving the organization of medical care. The Journal does not accept individual case reports or original articles involving bench laboratory or animal research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


