Mutational mechanisms in multiply relapsed pediatric acute lymphoblastic leukemia
IF 12.8
1区 医学
Q1 HEMATOLOGY
引用次数: 0
Abstract
Pediatric acute lymphoblastic leukemia (ALL) is marked by low mutational load at initial diagnosis, which increases at relapse. To determine which processes are active in (relapsed) ALL and how they behave during disease progression before and after therapy, we performed whole genome sequencing on 97 tumor samples of 29 multiply relapsed ALL patients. Mutational load increased upon relapse in 28 patients and upon every subsequent relapse in 22 patients. In addition to two clock-like mutational processes, we identified UV-like damage, APOBEC activity, reactive oxygen species, thiopurine-associated damage and an unknown therapy component as drivers of mutagenesis. Mutational processes often affected patients over longer time periods, but could also occur in isolated events, suggesting the requirement of additional triggers. Thiopurine exposure was the most prominent source of new mutations in relapse, affecting over half of the studied patients in first and/or later relapse and causing potential relapse-driving mutations in multiple patients. Our data demonstrate that multiple mutational processes frequently act in parallel as prominent secondary drivers with dynamic activity during ALL development and progression.
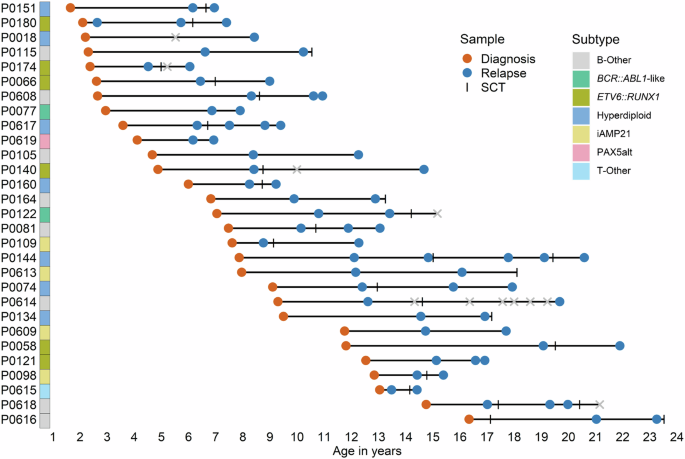
多次复发的小儿急性淋巴细胞白血病的突变机制。
小儿急性淋巴细胞白血病(ALL)的特点是初诊时突变负荷低,复发时突变负荷增加。为了确定哪些过程在(复发)ALL 中处于活跃状态,以及它们在治疗前后疾病进展过程中的表现,我们对 29 名多次复发的 ALL 患者的 97 份肿瘤样本进行了全基因组测序。结果显示,28 名患者的复发和 22 名患者的每次复发都会增加突变负荷。除了两个时钟样突变过程外,我们还发现紫外线样损伤、APOBEC活性、活性氧、硫嘌呤相关损伤和一种未知的治疗成分是诱变的驱动因素。突变过程对患者的影响往往持续较长时间,但也可能发生在孤立的事件中,这表明需要额外的触发因素。硫嘌呤暴露是复发中新突变的最主要来源,影响了半数以上首次复发和/或后期复发的患者,并在多名患者中引起潜在的复发驱动突变。我们的数据表明,在 ALL 的发生和发展过程中,多种突变过程经常作为显著的次要驱动因素并行作用,并具有动态活性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Leukemia
医学-血液学
CiteScore
18.10
自引率
3.50%
发文量
270
审稿时长
3-6 weeks
期刊介绍:
Title: Leukemia
Journal Overview:
Publishes high-quality, peer-reviewed research
Covers all aspects of research and treatment of leukemia and allied diseases
Includes studies of normal hemopoiesis due to comparative relevance
Topics of Interest:
Oncogenes
Growth factors
Stem cells
Leukemia genomics
Cell cycle
Signal transduction
Molecular targets for therapy
And more
Content Types:
Original research articles
Reviews
Letters
Correspondence
Comments elaborating on significant advances and covering topical issues
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


