Effectiveness and safety of monthly versus quarterly fremanezumab for migraine prevention: An Italian, multicenter, real-life study
Abstract
Background and Purpose
Fremanezumab, a monoclonal antibody targeting the calcitonin gene-related peptide for migraine prevention, is available in monthly (225 mg) and quarterly (675 mg) doses. Previous studies showed efficacy and safety for both regimens, but a real-life comparison is lacking. This study aimed to compare the effectiveness and safety of monthly and quarterly fremanezumab in a real-life setting.
Methods
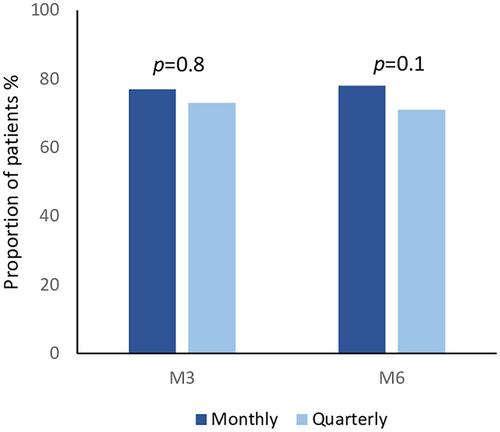
This Italian, prospective, multicenter study enrolled 95 migraine patients. During a 3-month treatment period, patients received either monthly or quarterly fremanezumab (49 monthly, 46 quarterly). A 6-month treatment period involved 79 patients (43 monthly, 36 quarterly). Monthly headache (MHD) and migraine days (MMD), number of days (AMD) and pills (AMP) of acute medication intake, and Headache Impact Test (HIT-6), Migraine Disability Assessment (MIDAS) test, and Numeric Rating Scale (NRS) scores were recorded at baseline and after 3 and 6 months of treatment. Adverse events (AEs), responder rates, and medication overuse were also investigated.
Results
Both monthly and quarterly treatments led to significant reductions in MMD, MHD, AMP, AMD, HIT-6, MIDAS, and NRS scores after 3 and 6 months. The monthly regimen exhibited a slightly greater reduction in MMD and MHD after the first quarter, with no significant difference observed after 6 months. The most common AE was transient injection-site reaction, without between-group differences. Responder rates and resolution of medication overuse did not significantly differ between the groups.
Conclusions
Both monthly and quarterly regimens were effective and safe, with a tendency for an advantage of the monthly regimen only in the first quarter of treatment.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


