Chiral Brønsted Acid-Catalyzed Intramolecular Asymmetric Dearomatization Reaction of Indoles with Cyclobutanones via Cascade Friedel–Crafts/Semipinacol Rearrangement
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
The highly efficient synthesis of chiral indolines fused with an azabicyclo[2.2.1]heptanone moiety is achieved by an asymmetric dearomatization reaction of indoles with cyclobutanones. A new chiral imidodiphosphorimidate (IDPi) catalyst is synthesized and exhibits extraordinary activity in promoting a cascade Friedel–Crafts/semipinacol rearrangement. Target molecules are prepared in good yields (up to 95%) with excellent enantioselectivity (up to 98% ee) with operational convenience. Combined experimental and computational studies provide detailed mechanistic insights into the energy landscape and origin of the stereochemical induction of the reaction.
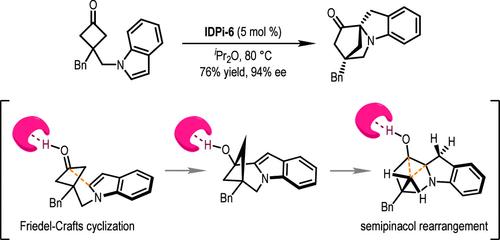
手性布氏酸催化的吲哚与环丁酮通过级联 Friedel-Crafts/Semipinacol 重排的分子内不对称脱芳烃反应
通过吲哚与环丁酮的不对称脱芳烃反应,高效合成了融合有氮杂双环[2.2.1]庚酮分子的手性吲哚啉。合成了一种新的手性亚胺二磷酰亚胺(IDPi)催化剂,该催化剂在促进弗里德尔-卡夫斯/塞米那醇级联重排方面表现出非凡的活性。目标分子的制备收率高(高达 95%),对映体选择性好(高达 98%ee),操作方便。实验和计算研究相结合,为了解反应的能量分布和立体化学诱导提供了详细的机理。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


