Inhibition of inflammatory osteoclasts accelerates callus remodeling in osteoporotic fractures by enhancing CGRP+TrkA+ signaling
IF 13.7
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Impaired callus remodeling significantly contributes to the delayed healing of osteoporotic fractures; however, the underlying mechanisms remain unclear. Sensory neuronal signaling plays a crucial role in bone repair. In this study, we aimed to investigate the pathological mechanisms hindering bone remodeling in osteoporotic fractures, particularly focusing on the role of sensory neuronal signaling. We demonstrate that in ovariectomized (OVX) mice, the loss of CGRP+TrkA+ sensory neuronal signaling during callus remodeling correlates with increased Cx3cr1+iOCs expression within the bone callus. Conditional knockout of Cx3cr1+iOCs restored CGRP+TrkA+ sensory neuronal, enabling normal callus remodeling progression. Mechanistically, we further demonstrate that Cx3cr1+iOCs secrete Sema3A in the osteoporotic fracture repair microenvironment, inhibiting CGRP+TrkA+ sensory neurons’ axonal regeneration and suppressing nerve–bone signaling exchange, thus hindering bone remodeling. Lastly, in human samples, we observed an association between the loss of CGRP+TrkA+ sensory neuronal signaling and increased expression of Cx3cr1+iOCs. In conclusion, enhancing CGRP+TrkA+ sensory nerve signaling by inhibiting Cx3cr1+iOCs activity presents a potential strategy for treating delayed healing in osteoporotic fractures.
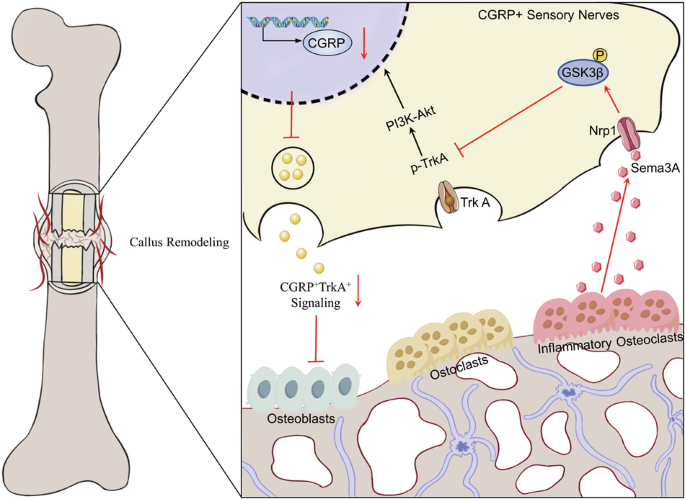
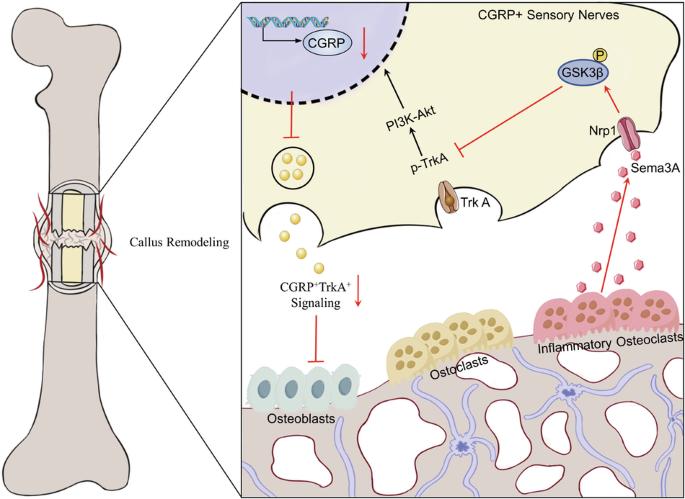
通过增强 CGRP+TrkA+ 信号,抑制炎性破骨细胞可加速骨质疏松性骨折的胼胝体重塑
胼胝体重塑受损是骨质疏松性骨折延迟愈合的重要原因,但其潜在机制仍不清楚。感觉神经元信号在骨修复中起着至关重要的作用。在这项研究中,我们旨在研究阻碍骨质疏松性骨折骨重塑的病理机制,尤其关注感觉神经元信号传导的作用。我们证明,在卵巢切除(OVX)小鼠中,胼胝体重塑过程中 CGRP+TrkA+ 感觉神经元信号的缺失与骨胼胝体中 Cx3cr1+iOCs 表达的增加相关。条件性敲除 Cx3cr1+iOCs 可恢复 CGRP+TrkA+ 感觉神经元,从而使胼胝体重塑过程正常进行。从机理上讲,我们进一步证明了 Cx3cr1+iOCs 在骨质疏松性骨折修复微环境中分泌 Sema3A,抑制 CGRP+TrkA+ 感觉神经元轴突再生,抑制神经-骨信号交换,从而阻碍骨重塑。最后,在人体样本中,我们观察到 CGRP+TrkA+ 感觉神经元信号的缺失与 Cx3cr1+iOCs 表达增加之间存在关联。总之,通过抑制 Cx3cr1+iOCs 的活性来增强 CGRP+TrkA+ 感觉神经信号传递是治疗骨质疏松性骨折延迟愈合的一种潜在策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell Death and Differentiation
生物-生化与分子生物学
CiteScore
24.70
自引率
1.60%
发文量
181
审稿时长
3 months
期刊介绍:
Mission, vision and values of Cell Death & Differentiation:
To devote itself to scientific excellence in the field of cell biology, molecular biology, and biochemistry of cell death and disease.
To provide a unified forum for scientists and clinical researchers
It is committed to the rapid publication of high quality original papers relating to these subjects, together with topical, usually solicited, reviews, meeting reports, editorial correspondence and occasional commentaries on controversial and scientifically informative issues.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


