Stakeholder-informed positivity thresholds for disease markers and risk scores: a methodological framework and an application in obstructive lung disease
IF 7.3
2区 医学
Q1 HEALTH CARE SCIENCES & SERVICES
引用次数: 0
Abstract
Objectives
A positivity threshold is often applied to markers or predicted risks to guide disease management. These thresholds are often decided exclusively by clinical experts despite being sensitive to the preferences of patients and general public as ultimate stakeholders.
Study Design and Setting
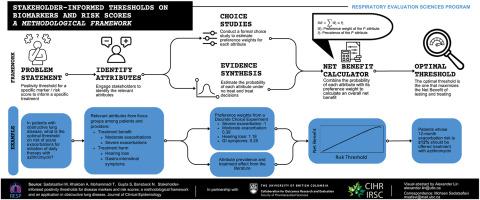
We propose an analytical framework for quantifying the net benefit (NB) of an evidence-based positivity threshold based on combining preference-sensitive (eg, how individuals weight benefits and harms of treatment) and preference-agnostic (eg, the magnitude of benefit and the risk of harm) parameters. We propose parsimonious choice experiments to elicit preference-sensitive parameters from stakeholders, and targeted evidence synthesis to quantify the value of preference-agnostic parameters. We apply this framework to maintenance of azithromycin therapy for chronic obstructive pulmonary disease using a discrete choice experiment to estimate preference weights for attribute level associated with treatment. We identify the positivity threshold on 12-month moderate or severe exacerbation risk that would maximize the NB of treatment in terms of severe exacerbations avoided.
Results
In the case study, the prevention of moderate and severe exacerbations (benefits) and the risk of hearing loss and gastrointestinal symptoms (harms) emerged as important attributes. Four hundred seventy seven respondents completed the discrete choice experiment survey. Relative to each percent risk of severe exacerbation, preference weights for each percent risk of moderate exacerbation, hearing loss, and gastrointestinal symptoms were 0.395 (95% confidence interval [CI] 0.338–0.456), 1.180 (95% CI 1.071–1.201), and 0.253 (95% CI 0.207–0.299), respectively. The optimal threshold that maximized NB was to treat patients with a 12-month risk of moderate or severe exacerbations ≥12%.
Conclusion
The proposed methodology can be applied to many contexts where the objective is to devise positivity thresholds that need to incorporate stakeholder preferences. Applying this framework to chronic obstructive pulmonary disease pharmacotherapy resulted in a stakeholder-informed treatment threshold that was substantially lower than the implicit thresholds in contemporary guidelines.
Plain Language Summary
Doctors often compare disease markers (such as laboratory results) or risk scores for a patient with cut-off values from guidelines to decide which patients need to be treated. For example, guidelines recommend that patients whose 10-year risk of heart attack is more than 10% be given statin pills. However, guidelines that recommend such treatment rules might not consider what matters most to patients (like how much they do not like side effects of the drugs). In this study, we propose a mathematical method where preferences of individuals on the trade-off between treatment benefits and harms can be used to determine the best treatment rule. We apply this method to the choice of antibiotic therapy for patients with lung airway diseases. We find that, given patient and public preferences on treatment benefit and risks, those with a 12% or more risk of experiencing a lung attack should receive antibiotic therapy. This patient-oriented cut-off is significantly lower than the cut-off values currently used by guidelines, which are in the 60%–70% range. We recommend applying this method whenever scientists must make recommendations on treatment rules where patient or public preferences might influence those rules.
由利益相关者提供信息的疾病标志物和风险评分阳性阈值:方法框架及在阻塞性肺病中的应用。
目的:阳性阈值通常适用于标记物或预测风险,以指导疾病管理。这些规则往往完全由临床专家决定,尽管对作为最终利益相关者的患者和公众的偏好非常敏感:我们提出了一个分析框架,在结合偏好敏感参数(如个人如何权衡治疗的益处和害处)和偏好无关参数(如益处大小和害处风险)的基础上,量化基于证据的阳性阈值的净益处。我们建议通过解析选择实验从利益相关者那里获得偏好敏感参数,并通过证据综合来量化偏好无关参数的价值。我们将这一框架应用于慢性阻塞性肺病(COPD)的阿奇霉素维持治疗,使用离散选择实验(DCE)估算与治疗相关的属性水平的偏好权重。我们确定了 12 个月中度或重度病情恶化风险的积极性阈值,该阈值可使治疗的净效益最大化,即避免重度病情恶化:在案例研究中,预防中度和重度病情加重(益处)以及听力损失和胃肠道症状风险(害处)成为重要属性。477 名受访者完成了 DCE 调查。相对于严重恶化风险的每个百分比,中度恶化、听力损失和胃肠道症状风险的每个百分比的偏好权重分别为 0.395(95%CI 0.338-0.456)、1.180(95%CI 1.071-1.201)和 0.253(95%CI 0.207-0.299)。净效益最大化的最佳阈值是治疗 12 个月中度或重度病情恶化风险≥12% 的患者:结论:所提出的方法可应用于许多情况,在这些情况下,目标是设计出需要纳入利益相关者偏好的积极性阈值。将这一框架应用于慢性阻塞性肺病药物治疗后,根据利益相关者的意见得出的治疗阈值大大低于当代指南中的隐性阈值。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Clinical Epidemiology
医学-公共卫生、环境卫生与职业卫生
CiteScore
12.00
自引率
6.90%
发文量
320
审稿时长
44 days
期刊介绍:
The Journal of Clinical Epidemiology strives to enhance the quality of clinical and patient-oriented healthcare research by advancing and applying innovative methods in conducting, presenting, synthesizing, disseminating, and translating research results into optimal clinical practice. Special emphasis is placed on training new generations of scientists and clinical practice leaders.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


