Synergistic effects of combined BET and FAK inhibition against Vestibular Schwannomas in NF2-related Schwannomatosis
IF 7.3
1区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
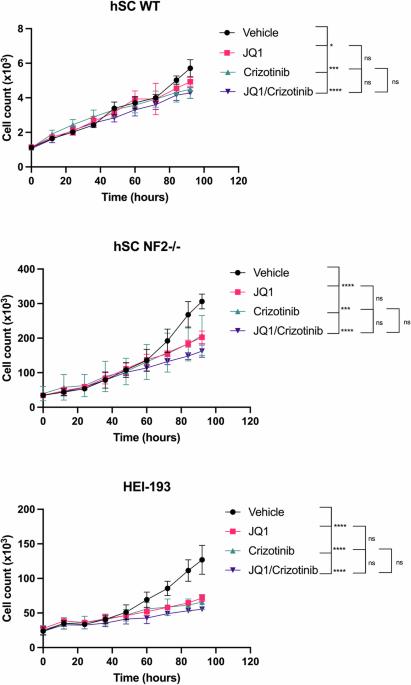
Neurofibromatosis type 2 (NF2) is a rare disorder that causes vestibular schwannomas (VS), meningiomas and ependymomas. To date, there is no FDA approved drug-based treatment for NF2. We have previously identified that BET inhibition can selectively reduce growth of the NF2-null schwannoma and Schwann cells in vitro and tumorigenesis in vivo and, separately, reported that inhibition of Focal Adhesion Kinase 1 (FAK1) via crizotinib has antiproliferative effects in NF2-null Schwann cells. The current study was aimed at determining whether combined BET and FAK inhibition can synergize and to identify the mechanisms of action. A panel of normal and NF2-null Schwann and schwannoma cell lines were used to characterize the effects of combined BET and FAK inhibition in vitro and in vivo using pharmacological and genetic approaches. The mechanism of action was explored by chromatin immunoprecipitation, ChIP-PCR, western blotting, and functional approaches. We find that combined BET and FAK inhibition are synergistic and inhibit the proliferation of NF2-null schwannoma and Schwann cell lines in vitro and in vivo, by arresting cells in the G1/S and G2/M phases of the cell cycle. Further, we identify the mechanism of action through the downregulation of FAK1 transcription by BET inhibition, which potentiates inhibition of FAK by 100-fold. Our findings suggest that combined targeting of BET and FAK1 may offer a potential therapeutic option for the treatment of NF2-related schwannomas.
联合抑制 BET 和 FAK 对 NF2 相关性前庭神经丛神经瘤的协同作用
神经纤维瘤病 2 型(NF2)是一种罕见疾病,可导致前庭裂神经瘤(VS)、脑膜瘤和上皮瘤。迄今为止,美国食品及药物管理局尚未批准基于药物的 NF2 治疗方法。我们以前曾发现,抑制 BET 可选择性地减少 NF2 基因缺失的裂神经瘤和许旺细胞在体外的生长和在体内的肿瘤发生,并分别报道了通过克唑替尼抑制 Focal Adhesion Kinase 1 (FAK1) 对 NF2 基因缺失的许旺细胞具有抗增殖作用。本研究旨在确定 BET 和 FAK 联合抑制是否能产生协同作用,并确定其作用机制。研究人员使用一组正常和 NF2 基因无效的许旺细胞系和裂隙瘤细胞系,通过药理学和遗传学方法,对 BET 和 FAK 联合抑制在体外和体内的作用进行了表征。通过染色质免疫共沉淀、ChIP-PCR、Western 印迹和功能方法探讨了其作用机制。我们发现,联合抑制 BET 和 FAK 具有协同作用,通过使细胞停滞在细胞周期的 G1/S 和 G2/M 期,在体外和体内抑制了 NF2 基因缺失的裂隙瘤和许旺细胞系的增殖。此外,我们还确定了通过抑制 BET 下调 FAK1 转录的作用机制,该机制可使对 FAK 的抑制增强 100 倍。我们的研究结果表明,联合靶向 BET 和 FAK1 可为治疗 NF2 相关裂隙瘤提供一种潜在的治疗方案。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Oncogene
医学-生化与分子生物学
CiteScore
15.30
自引率
1.20%
发文量
404
审稿时长
1 months
期刊介绍:
Oncogene is dedicated to advancing our understanding of cancer processes through the publication of exceptional research. The journal seeks to disseminate work that challenges conventional theories and contributes to establishing new paradigms in the etio-pathogenesis, diagnosis, treatment, or prevention of cancers. Emphasis is placed on research shedding light on processes driving metastatic spread and providing crucial insights into cancer biology beyond existing knowledge.
Areas covered include the cellular and molecular biology of cancer, resistance to cancer therapies, and the development of improved approaches to enhance survival. Oncogene spans the spectrum of cancer biology, from fundamental and theoretical work to translational, applied, and clinical research, including early and late Phase clinical trials, particularly those with biologic and translational endpoints.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


